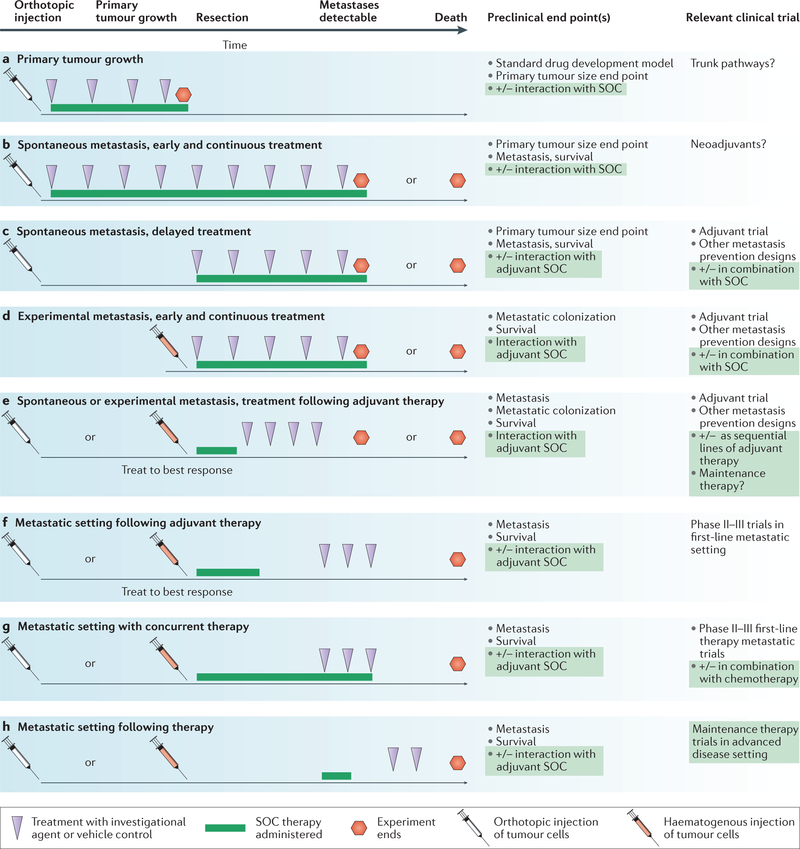
Figure 4 |. Meaningful incorporation of preclinical metastasis models into drug development.
Potential experimental designs for an antimetastatic investigational agent are mapped along a timeline. Tumour cell injection can either be orthotopic, into the tissue of origin (white needles) or haematogenous for experimental metastasis (red needles). The investigational agent (purple arrowheads) can be delivered for all or part of the assay; optimally it should reflect the oral or intravenous dosing to be used in the clinic. Standard of care (SOC) therapy (green line), at a clinically achievable dose, can be added before, concurrent with or after the investigational agent and can use agents approved in the adjuvant or metastatic setting. a | Standard drug development uses primary tumour growth as an end point, which completely neglects the metastatic process. b–e | Model systems with different degrees of applicability to adjuvant setting trials preventing metastatic colonization. f–h | Model systems with different applicabilities to metastatic setting trials. End points include the number and size of metastases by histology and imaging, pharmacodynamic markers of drug activity or tumour biology, and survival.