Abstract
Background
Plasma ammonia is commonly measured in the diagnostic evaluation of hospitalized newborns, but reference values are not well defined.
Methods
We prospectively enrolled newborns admitted to the level III/IV neonatal intensive care unit and level II intermediate special care nursery from January 2017 to January 2018. Infants with inborn errors of metabolism or liver disease were excluded. Plasma ammonia concentrations were measured once within the first week of life and evaluated by sex, gestational age, timing of the draw, blood collection method, and type of nutrition. Reference intervals were calculated.
Results
127 neonates were included; one third (34%) were term infants born at ≥37 weeks gestation, and two thirds (66%) were born preterm at <37 weeks gestation. Median plasma ammonia concentrations were 32 μmol/L (range <10 to 86 μmol/L). Median ammonia concentrations were higher among preterm compared to term infants (35 vs. 28 μmol/L, p = 0.0119), and term female compared to term male infants (34 vs. 26 μmol/L, p = 0.0228). There was no difference in median ammonia concentrations between female and male preterm infants, based on gestational age within the preterm group, timing of the blood draw, presence of hyperbilirubinemia, blood collection method, or type of nutritional intake.
Conclusions
Plasma ammonia concentrations among newborns are higher than the expected adult concentrations and may vary by gestational age and sex. Blood collection method, type of nutrition, hyperbilirubinemia, and timing of the draw do not impact concentrations. We propose a reference limit of ≤82 μmol/L for newborns less than one week of age.
Keywords: Ammonia, newborn, inborn error of metabolism
Impact Statement
Plasma ammonia concentrations are commonly measured in newborns, as conditions associated with hyperammonemia, such as inborn errors of metabolism, can present similarly to sepsis or other illnesses in the early postnatal period. Expected values for newborns are not well defined, so clinicians must rely on adult reference intervals when interpreting ammonia concentrations in newborns. Our study established plasma ammonia reference intervals among term and preterm neonates without inborn errors of metabolism or underlying liver disease. We also evaluated the impact of sex, gestational age, neonatal nutrition, hyperbilirubinemia, blood collection method, and timing of the draw on ammonia values.
Introduction
Inborn errors of metabolism presenting in the neonatal period are rare but potentially life-threatening conditions that require a high index of suspicion for neonatal providers, as early diagnosis and treatment is crucial in preventing morbidity and mortality (1, 2). Symptoms in affected neonates are often nonspecific, including poor feeding, vomiting, lethargy, tachypnea or apnea, and are difficult to distinguish from other more common problems, such as sepsis (2).
Hyperammonemia is observed in a number of inborn errors of metabolism, including urea cycle defects, organic acidemias, and disorders of fatty-acid oxidation (1). Measurement of plasma ammonia is part of the basic workup for a neonate with a suspected metabolic condition (2, 3). However, reference intervals for ammonia among neonates, especially premature neonates, are not well defined. Most laboratories, including ours, do not report a reference interval (RI) for ammonia based on age, with RIs generally derived for healthy adults. Prior studies suggest that ammonia concentrations in newborns may be higher than those observed in older infants, children, and adults (4–8), and decline after 1 to 2 weeks following birth (4, 5, 7, 9). Plasma ammonia concentrations also may vary depending upon gestational age (9). We sought to establish RIs for ammonia among term and preterm neonates by prospectively enrolling infants admitted to the level III/IV neonatal intensive care unit (NICU) and level II intermediate special care nursery (ISCN).
Methods
Reference sample group
All neonates admitted to the NICU or ISCN at Mayo Clinic in Rochester, MN between January 2017 and January 2018 were eligible to be included in this study. Infants whose parent or legal guardian gave informed consent for study participation within 72 h of birth were prospectively enrolled. The medical records of infants and their mothers were reviewed to gather demographic information, delivery information, comorbidities, and clinical findings. Enrolled infants with a positive screen for a metabolic disorder on the Minnesota Newborn Screening (MNS) confirmed in subsequent testing, or those with liver disease diagnosed during their hospitalization were excluded. The MNS includes over 50 disorders including amino acid disorders, fatty acid oxidation disorders, organic acid disorders, endocrine disorders, lysosomal disorders, hemoglobinopathies, and other congenital disorders (https://www.health.state.mn.us/people/newbornscreening/program/newbornscreeningpanel.html). All neonates were assessed for hyperbilirubinemia, as recommended by the American Academy of Pediatrics (10). Total serum bilirubin was measured with a modified Diazo method using the Roche Total Bilirubin reagent (Roche Diagnostics, Indianapolis, IN) run on Roche Cobas c501 or c701 instruments. Determination regarding the need for phototherapy was made by the treating physicians according to the nomogram of hour-specific serum bilirubin values. This study was approved by Mayo Clinic’s Institutional Review Board.
Blood sampling and ammonia measurements
A venous blood sample of at least 0.5 mL was collected in an ethylene diamine tetraacetic acid (EDTA) tube, obtained by venipuncture or aspiration from an umbilical venous catheter (UVC). Specimens were placed on ice immediately after collection, and centrifuged at refrigerated temperature (2°-6°C) to separate out the plasma, which was kept on ice until analyzed. Plasma ammonia concentrations were measured by bromophenol blue photometry (VITROS® 350, Ortho Clinical Diagnostics, Raritan, NJ). This method demonstrates coefficients of variation of 15.5% at 23 μmol/L, 5.1% at 83 μmol/L, and 3.6% at 249 μmol/L. The expected normal range for adults according to the manufacturer’s package insert is ≤30 μmol/L and was verified according to CLSI EP28-A3c (11).
Statistical analysis
Reference limits and associated 90% confidence intervals were calculated as 2.5th and 97.5th percentiles to derive the reported central 95% RIs from nonparametric (overall) and log transformed parametric (subgroup) analysis using EP Evaluator® (Data Innovations, Burlington, VT). Confidence ratios represent the ratio of average confidence interval width to RI width to ascertain inclusion of sufficient reference values, with values <0.20 preferred (11). The program’s partitioning test was used to determine whether the RI would benefit from being calculated separately for subgroups with Z max >2.2 and SD Ratio >1.5 suggested critical limits. Descriptive summaries were reported as medians (minimums, maximums) for continuous variables, and as frequencies and percentages for categorical variables. Normality assumption for ammonia concentration as an outcome of interest was assessed using the Kolmogorov-Smirnov test of normality. Comparisons of ammonia concentrations within various subgroups of categorical variables were performed using Kruskall-Wallis or Wilcoxon rank sum tests, as appropriate. All tests were two sided; P values <0.05 were considered statistically significant. Analysis was performed using SAS software version 9.4 (SAS Inc, Cary, NC).
Results
One hundred and forty-eight neonates were enrolled in the study over one year. Twenty-one infants were excluded, 13 for lack of sample collection after enrollment, and 8 for collection of sample from an arterial source. The remaining 127 infants were included in the study. All had normal MNS results or normal follow up testing. None of the infants were known to have underlying liver disease. One third (34%) of infants were born at term (≥37 weeks gestation), and two-thirds (66%) were born preterm (<37 weeks gestation). The preterm group included infants born at 34-36 weeks (n = 54, 42%), 29-33 weeks (n = 25, 20%), and <29 weeks (n = 5, 4%). Seventy-three (57%) infants were male, and 54 (43%) female. Among female infants, 13 (24%) were born at term, and 41 (76%) preterm. Among male infants, 30 (41%) were born at term, and 43 (59%) preterm.
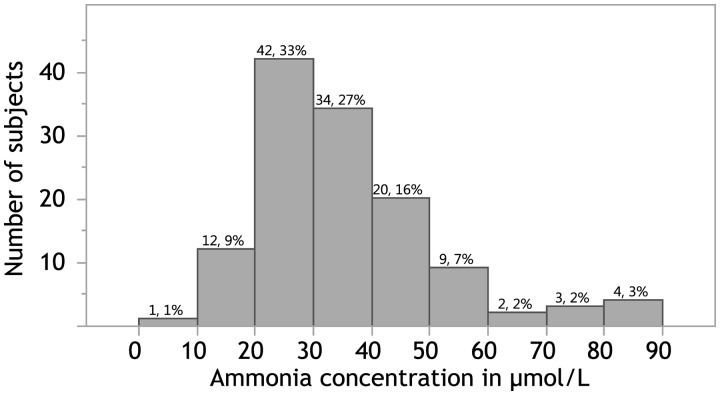
Blood was drawn between the day of birth and day-of-life six. The median age at blood draw was 39.3 h. The distribution of ammonia concentrations is shown in Fig. 1. Although approaching a normal distribution, values were not normally distributed when assessed with the Kolmogorov-Smirnov test of normality. Overall median ammonia concentrations were 32 μmol/L, with a range of <10 to 86 μmol/L and a calculated central 95% RI of 12–82 μmol/L. Table 1 shows ammonia concentrations by subgroups. RI analysis was performed on subgroups that demonstrated statistically significant differences. The median ammonia concentrations were higher among female compared to male infants (35 vs. 29 μmol/L, P = 0.0079), and among preterm compared to term infants (35 vs. 28, P = 0.0119). When comparing term and preterm infants by sex, there was a difference between median ammonia concentrations of female and male term infants (34 vs. 26 μmol/L, P = 0.0228), but not among female and male preterm infants (35 vs. 33 μmol/L, P = 0.3448). The calculated RIs for these subgroups were subjected to a partitioning test for gestational age and sex. Notably, they did not reach significance based on both calculated Z max and SD Ratio. There was no statistical difference in median ammonia concentrations based on gestational age within the preterm group, timing of the blood draw, method of blood collection, presence of hyperbilirubinemia requiring phototherapy, or type of nutritional intake (comparing total parenteral nutrition with enteral nutrition or a combination of both, as well as comparing those receiving formula only, breastmilk only, or a combination of formula and breastmilk).
Fig. 1.
Distribution of ammonia concentrations in μmol/L among all 127 tested neonates. Histogram displaying the distribution of ammonia concentrations among all tested neonates in 10 μmol/L increments. The numbers above the columns represent the number of infants and percentage of values within each column, respectively.
Table 1.
Median ammonia concentrations among different subgroups of neonates.
| Number of neonates (%) | 2.5th and 97.5th reference limits (90% CI) µmol/L | Confidence ratioa | Z maxb | SD Ratiob | Median ammonia concentration in µmol/L (minimum, maximum) | P value | |
|---|---|---|---|---|---|---|---|
| All neonates | 127 (100%) | 12 (10-15) | 0.14 | 32 (<10, 86) | - | ||
| 82 (72-86) | |||||||
| Sex | 1.6 | 1.4 | 0.0079 | ||||
| Male | 73 (57%) | 11 (9-13) | 0.22 | 29 (<10, 86) | |||
| 77 (66-91) | |||||||
| Female | 54 (43%) | 20 (17-22) | 0.22 | 35 (18, 82) | |||
| 65 (57-73) | |||||||
| Gestational age at birth | 1.7 | 1.0 | 0.0119 | ||||
| Term (≥37 weeks) | 43 (34%) | 13 (11-15) | 0.27 | 28 (10, 86) | |||
| 66 (55-79) | |||||||
| Preterm (<37 weeks) | 84 (66%) | 14 (12-16) | 0.19 | 35 (<10, 82) | |||
| 79 (69-90) | |||||||
| Collection method | n/a | n/a | 0.2610 | ||||
| Venipuncture | 80 (63%) | n/a | 33 (<10, 86) | ||||
| Umbilical venous catheter | 47 (37%) | n/a | 31 (12, 74) | ||||
| Collection time | n/a | n/a | 0.2820 | ||||
| <24 hours of life | 7 (6%) | n/a | 31 (12, 57) | ||||
| 24-48 hours of life | 67 (53%) | 33 (<10, 82) | |||||
| 48-72 hours of life | 38 (30%) | 28 (14, 86) | |||||
| >72 hours of life | 15 (12%) | n/a | 38 (14, 61) | ||||
| Feeding at the time of ammonia draw | n/a | n/a | 0.5789 | ||||
| Total parenteral nutrition only | 12 (9%) | n/a | 35 (10, 72) | ||||
| Enteral nutrition onlyc | 80 (63%) | n/a | 32 (14, 86) | ||||
| Both parenteral and enteral nutrition | 35 (28%) | n/a | 32 (<10, 55) | ||||
| Hyperbilirubinemia requiring phototherapy | n/a | n/a | 0.3798 | ||||
| Yes | 62 (49%) | n/a | 33 (<10, 86) | ||||
| No | 65 (51%) | n/a | 31 (10, 82) |
Confidence ratio <0.20 is desirable.
Either Z max >2.2 or SD Ratio >1.5 suggests a separate reference interval (RI) may be warranted.
Includes formula and breastmilk by oral intake and tube feedings. Some infants in this group were receiving dextrose-containing intravenous fluids, but not parenteral amino acid or lipid formulations.
Discussion
Our findings are in accordance with studies that report higher ammonia concentrations among newborns (4–8). Also in keeping with a prior study (9), we found higher median ammonia concentrations among preterm newborns, among whom median ammonia concentrations did not vary based on gestational age. We also noted a difference between median ammonia concentrations of female and male term infants. Usmani et al. noted no difference in ammonia concentrations based on sex, but only evaluated preterm infants <36 weeks, a population in which we also saw no difference between female and male infants (9). Clemmens et al. described no significant difference in ammonia concentrations based on sex, but reported higher values among female compared to male term infants (8). We note that the statistically significant differences we detected in median ammonia concentrations among term and preterm infants, as well as female and male infants, may not be of clinical significance, as supported by the non-significant Z max and SD Ratio for subgroup RI analysis. For this reason, we propose a single reference limit of ≤82 μmol/L for all newborns less than one week of age as the lower reference limit (12 μmol/L) approaches the lowest ammonia concentration that can be detected reliably (10 μmol/L) and distinction of low ammonia concentration is of little clinical value.
Previous studies suggested higher ammonia concentrations among infants receiving certain formulas (12) and among those receiving total parenteral nutrition (13, 14). We did not find a difference in ammonia concentrations based on these variables. Similarly, elevated concentrations of ammonia were described in infants with hyperbilirubinemia in one older study (7), an association our study and another small study (8) did not confirm.
This study has several limitations. Ammonia RIs were calculated from a reference population of infants admitted to the hospital, which represents the most common clinical scenario where ammonia is measured. Transference of this RI should be done with caution to non-hospitalized neonatal populations, as well as other testing platforms (15). Blood was collected only once from each infant, and therefore trends in ammonia concentrations over time could not be studied. Also, all blood samples were collected within the first week of life, therefore no estimates of the RI for older infants were made.
In summary, ammonia concentrations among neonates within the first week of life were higher than generally accepted for healthy adults. Our study established a 97.5th percentile plasma ammonia reference limit of ≤82 μmol/L for hospitalized infants less than one week of age.
Acknowledgments
We thank the Department of Laboratory Medicine and Pathology at Mayo Clinic for generously supporting this study through a Collaborative Award, and the Research and Innovation Office for helping us execute this study. We thank the National Institutes of Health for supporting our hyperammonemia work through AI 150649. We also thank Christopher T. Dolan for performing the RI calculations, Jayawant N. Mandrekar, Ph.D. for his help with statistical data analysis, and the staff in the neonatal units for their help with sample collection.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
T. Madigan, statistical analysis, provision of study material or patients; W. Carey, provision of study material or patients.
Authors' Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest: Employment or Leadership: R. Patel, Mayo Clinic. Consultant or Advisory Role: R. Patel, Curetis, Qvella, St. Jude, Beckman Coulter, Morgan Stanley, Heraeus Medical GmbH, CORMATRIX, Specific Technologies, Diaxonit, Selux Dx, GenMark Diagnostics, LBT Innovations Ltd, PathoQuest, Genentech (monies are paid to Mayo Clinic). R. Patel has served on an Actelion data monitoring board. Stock Ownership: None declared. Honoraria: R. Patel, the NBME, Up-to-Date, and the Infectious Diseases Board Review Course. Research Funding: R. Patel, CD Diagnostics, BioFire, Curetis, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, Allergan, The Medicines Company. The Department of Laboratory Medicine and Pathology at Mayo Clinic supported this study through a Collaborative Award. The National Institutes of Health supported hyperammonemia work through AI 150649.
Expert Testimony: None declared. Patents: R. Patel, a patent on Bordetella pertussis/parapertussis PCR issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued.
Other Remuneration: R. Patel, travel reimbursement from ASM and IDSA and an editor's stipend from ASM and IDSA.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
REFERENCES
- 1. Leonard JV, Morris AA.. Inborn errors of metabolism around time of birth. Lancet 2000;356:583–7. [DOI] [PubMed] [Google Scholar]
- 2. Burton BK. Inborn errors of metabolism in infancy: a guide to diagnosis. Pediatrics 1998; 102(6):E69. [DOI] [PubMed] [Google Scholar]
- 3. Ellaway CJ, Wilcken B, Christodoulou J.. Clinical approach to inborn errors of metabolism presenting in the newborn period. J Paediatr Child Health 2002;38:511–7. [DOI] [PubMed] [Google Scholar]
- 4. Cooke RJ, Jensen RL.. Plasma ammonia concentration during the 1st 6 postnatal months, as measured with an ammonium-selective electrode. Clin Chem 1983;29:1563–4. [PubMed] [Google Scholar]
- 5. Beddis IR, Hughes EA, Rosser E, Fenton JC.. Plasma ammonia levels in newborn infants admitted to an intensive care baby unit. Arch Dis Child 1980;55:516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diaz J, Tornel PL, Martinez P.. Reference intervals for blood ammonia in healthy subjects, determined by microdiffusion. Clin Chem 1995;41:1048. [PubMed] [Google Scholar]
- 7. McGovern JJ, McDermott WV Jr, McGovern MN, Russel M, McGrath E, Holtz A.. Ammonia metabolism in normal newborn infants and those with idiopathic hyperbilirubinemia. Pediatrics 1959;23:1160–7. [PubMed] [Google Scholar]
- 8. Clemmens RL, Shear SB, Bessman SP.. Ammonia in the blood in newborn infants. Pediatrics 1958;21:22–6. [PubMed] [Google Scholar]
- 9. Usmani SS, Cavaliere T, Casatelli J, Harper RG.. Plasma ammonia levels in very-low-birth-weight preterm infants. J Pediatr 1993;123:797–800. [DOI] [PubMed] [Google Scholar]
- 10. Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF.. Hyperbilirubinemia in the newborn infant > or =35 weeks' gestation: an update with clarifications. Pediatrics 2009;124:1193–8. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute (CLSI). Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; EP28-A3c. Wayne (PA); 2010.
- 12. Raiha NC, Heinonen K, Rassin DK, Gaull GE.. Milk protein quantity and quality in low-birthweight infants: I. Metabolic responses and effects on growth. Pediatrics 1976;57:659–84. [PubMed] [Google Scholar]
- 13. Johnson JD, Albritton WL, Sunshine P.. Hyperammonemia accompanying parenteral nutrition in newborn infants. J Pediatr 1972;81:154–61. [DOI] [PubMed] [Google Scholar]
- 14. Heird WC, Nicholson JF, Driscoll JM Jr, Schullinger JN, Winters RW.. Hyperammonemia resulting from intravenous alimentation using a mixture of synthetic l-amino acids: a preliminary report. J Pediatr 1972;81:162–5. [DOI] [PubMed] [Google Scholar]
- 15. Markus C, Metz M.. Comparison of plasma ammonia results from seven different automated platforms in use throughout Central Australia. Clin Biochem 2017;50:331–5. [DOI] [PubMed] [Google Scholar]