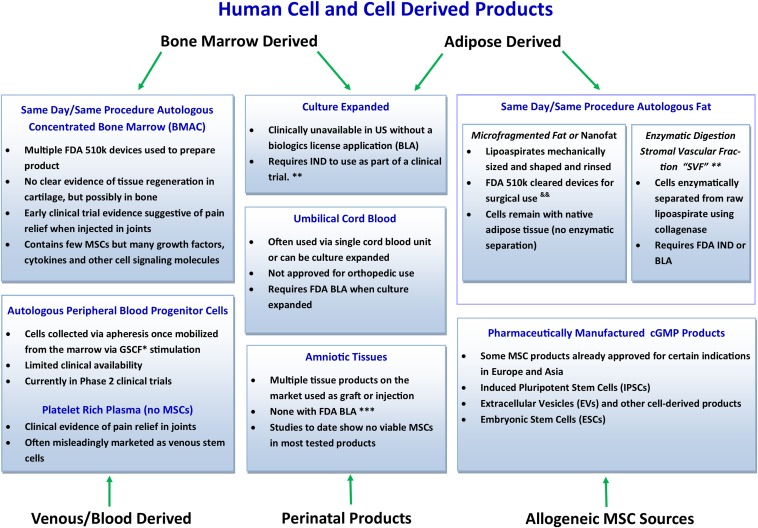
Fig. 1.
Conceptual representation of current human cell and cell-derived products for orthopaedic use. *GCFS = granulocyte colony-stimulating factor. **Limited access worldwide, although some options are available in countries with little/no regulation. &&Practice in the United States requires adherence to minimal manipulation, not more than rinsing, sizing, and shaping, as outlined in the U.S. FDA Same Surgical Procedure Exception (SSPE). ***Multiple devices are available that utilize enzymatic digestion of SVF cells from adipocytes. Considered by the FDA to be more than minimal manipulation and thus outside the scope of SSPE; would require FDA IND or BLA to comply with current U.S. regulatory framework. BMAC = bone marrow aspirate concentrate, FDA = U.S. Food and Drug Administration, MSCs = mesenchymal stem cells, IND = Investigational New Drug, and cGMP = current good manufacturing process.