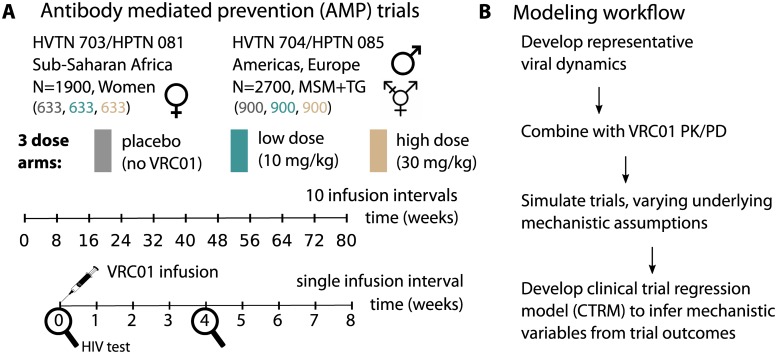
Fig 1. The model implementation of the Antibody Mediated Prevention (AMP) trials.
A) Two parallel trials in African women and North American MSM and TG individuals. Each trial contains 3 equally populated arms, placebo (gray), 10 mg/kg infusions (teal), and 30 mg/kg infusions (tan). Infusions occur every 8 weeks, for a total of 80 weeks and HIV testing occurs every 4 weeks. We refer to an 8 week interval as a ‘dosing interval’. B) The model workflow outlines the results.