Abstract
BACKGROUND AND PURPOSE: Coil embolization of berry aneurysms is a relatively new treatment whose long-term efficacy has yet to be established. The purpose of this study was, first, to attempt to identify factors that might be important in predicting success both at the time of treatment and at the time of follow-up angiography, and, second, to study changes in the aneurysm between treatment and follow-up to determine the frequency of these changes.
METHODS: The pretreatment, posttreatment, and follow-up angiograms of the first 63 aneurysms (in 58 patients) treated at our institution between June 1992 and April 1995 were analyzed, and the percentage of occlusion of each aneurysm was calculated. The size of any rest was noted for the posttreatment and follow-up angiograms. Treatment success was defined as a residue of less than 2 mm. Aneurysms were said to have changed if the percentage of occlusion had altered by more than 2.5% or if the difference in rest size was greater than 0.25 mm. Possible factors influencing primary and follow-up success rates were correlated against these calculations.
RESULTS: Success rates at treatment and follow-up were 71% and 65%, respectively. No change occurred in 41% of aneurysms, and 20% had a decrease in size of the residue. Twenty-eight percent had coil compaction, and 11% had aneurysmal growth. Neck size was the only significant variable in primary treatment success. Success at follow-up correlated significantly with neck size, initial treatment success, vasospasm at the time of treatment, and clinical presentation.
CONCLUSION: Best long-term angiographic results are obtained when the primary treatment is successful, when the aneurysm is small and narrow-necked, when the acutely ruptured aneurysm is treated within 15 days of ictus, and with anterior communicating and basilar-tip aneurysms.
Endovascular treatment of berry aneurysms with Guglielmi detachable coils (GDCs) is generally associated with a lower rate of complete obliteration of the aneurysmal lumen than is treatment with surgical clipping. Early results of this form of treatment show promise, both clinically in terms of the safety and prevention of rehemorrhage in the short term, and anatomically, in terms of obliteration of the aneurysmal lumen (1–4).
This aneurysmal residue, or “rest,” in coiled aneurysms may enlarge by coil compaction, or the aneurysm may grow. The rest may also spontaneously decrease in size or disappear at the time of angiographic follow-up. Additionally, even completely coiled aneurysms may undergo coil compaction. Follow-up angiography, therefore, is usually recommended to determine the final anatomic status of the aneurysm as successfully treated or not. Although aneurysmal neck size has been shown to be important in predicting successful obliteration of the lumen at the time of treatment, less is known of the factors that are important in predicting success at the time of follow-up angiography.
Aneurysmal recurrence after surgical treatment is usually measured in terms of rehemorrhage rates, rather than by review imaging. This rehemorrhage is generally accepted to be subsequent to incomplete occlusion of the aneurysmal lumen (5–11), and a similar mechanism is probably responsible for rehemorrhage from aneurysms treated by the endovascular route (2–12). The completeness of luminal occlusion after coiling, therefore, is an important predictor of aneurysmal recurrence (4, 12). Factors observed to influence the degree of luminal occlusion after coil embolization include the aneurysmal neck size, the luminal size, and possibly the degree of luminal packing (1, 13–15).
Aneurysmal lumens that are incompletely obliterated surgically may enlarge and rehemorrhage, remain unchanged, or undergo spontaneous thrombosis (16). Some of these changes in the aneurysmal residue have also been observed at the time of follow-up angiography in aneurysms treated by the endovascular route and may occur more frequently in this group (1, 2, 4). Because of this potential for change in coiled aneurysms, a follow-up angiogram is necessary for objective prognostication, particularly at this stage of a new technique's development. Factors that influence changes in the aneurysmal residue between treatment and follow-up and in the success rates at the time of follow-up have been less well studied.
The purpose of our study was to determine which factors were most influential in determining success of embolization at the time of initial treatment and at the time of follow-up. We also evaluated the morphologic changes in the residual aneurysmal lumen between treatment and follow-up.
Methods
Patients were selected for study according to endoluminal occlusion of one or more berry aneurysms using the GDC system with preservation of the parent artery lumen and according to digital or analog angiography before treatment, at the completion of treatment, and at follow-up (6 months later or at recurrence of symptoms), together with clinical charts and cross-sectional imaging.
From each of the three angiographic studies, one image was chosen because it most closely reflected the anatomy of the aneurysm and its neck and because it reproduced as closely as possible the projections in the other studies. This projection was usually the one used during embolization. Each image, in turn, was then projected onto calibrated paper (1-mm squares) and superimposed on the previous image, using variable magnification to allow for differences in radiologic magnification between studies. A tracing was then made of the untreated aneurysm and its neck, the treated aneurysm (with any residual lumen), and the aneurysm at follow-up (Figs 1 and 2).
fig 1.
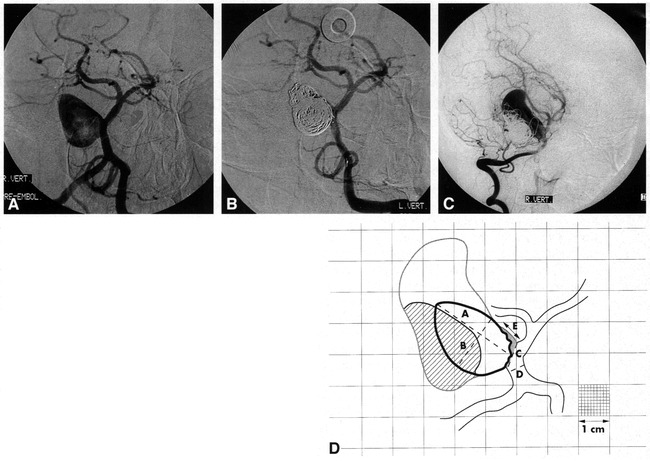
Aneurysmal growth.
A, Pretreatment angiogram of a large, wide-necked, basilar sidewall aneurysm occurring as a mass lesion. This image is traced onto calibrated paper, with the margins of the aneurysmal lumen delineated in D as the solid black line. From the tracing, and using the linear reference value “D,” luminal size “A,” luminal width “B,” and neck size “C” are calculated (as shown in D).
B, Posttreatment angiogram after coil placement. This image was used to trace the margin of the coil ball in D and to calculate the percentage of occlusion (94%) and rest size (“E ” in D).
C, Follow-up angiogram at 10 months shows aneurysmal growth. After tracing this image, the new aneurysmal lumen is delineated by the outer gray line in D. The compacted coil-ball mass is now delineated by the oblique lines.
D, Traced superimposed representations of A–C (artist's rendition). For clarity, only the 1-cm grid lines are portrayed (a portion of the figure shows the 1-mm calibrations as a reference). The shaded area at the aneurysmal neck represents the residual uncoiled aneurysm. The area filled with oblique lines represents the compacted coil-ball mass. “D” is the linear reference value for the calculation of “A” (maximum luminal size, 20 mm), “B” (maximum luminal width, 12.7 mm), “C” (neck size, 4.8 mm), and “E” (rest size after treatment, 5.2 mm).
fig 2.
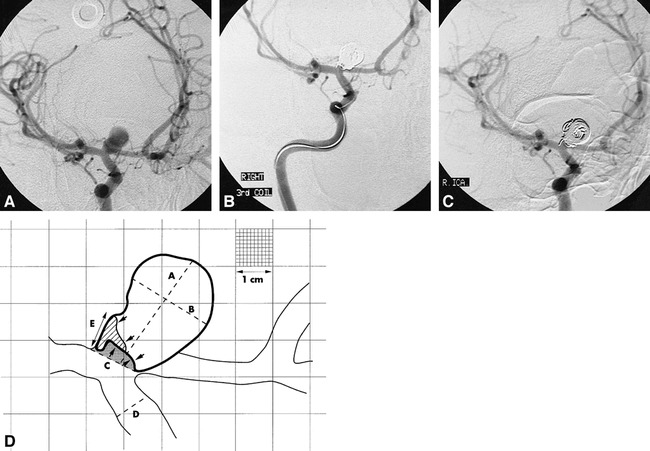
Coil compaction.
A, Pretreatment angiogram.
B, Posttreatment angiogram.
C, Follow-up angiogram at 7 months of a wide-necked (4.6 mm) carotid termination aneurysm.
D, Traced image (artist's rendition) derived from A, B, and C. The shaded area represents the residual uncoiled lumen at the time of treatment. The area filled by oblique lines is the additional lumen exposed by coil compaction. This is an example of initial treatment success (rest size < 2 mm) converting to failure at follow-up (rest size = 3.4 mm). For clarity, only the 1-cm calibrations have been rendered, with a portion of the figure showing the full 1-mm grid lines. “D” = linear reference diameter; “A” = luminal size (12.2 mm); “B” = luminal width (8.6 mm); “C” = neck size (4–6 mm); “E” = rest size at follow-up (3.4 mm).
From the superimposed traced images, the following measurements were performed: the percentage of occlusion of the aneurysm was calculated for the posttreatment and follow-up studies by counting squares; linear measures of maximum luminal size and neck diameter (calculated from the pretreatment tracing) were obtained using a reference vessel as described by Zubillaga et al (15); and the size of the aneurysmal residue (rest), if present, was calculated for the posttreatment and follow-up angiograms, using the same linear reference (15). The rest was defined as the small segment at the base of the aneurysm proximal to the coil ball that was still filled with contrast medium on postoperative and follow-up angiography and, therefore, was morphologically similar to the surgical rest defined by Mennonna et al (17). The rest dimension was calculated in millimeters as the longest length of exposed aneurysmal wall that was not protected by the coil-ball mass (Fig 3).
fig 3.
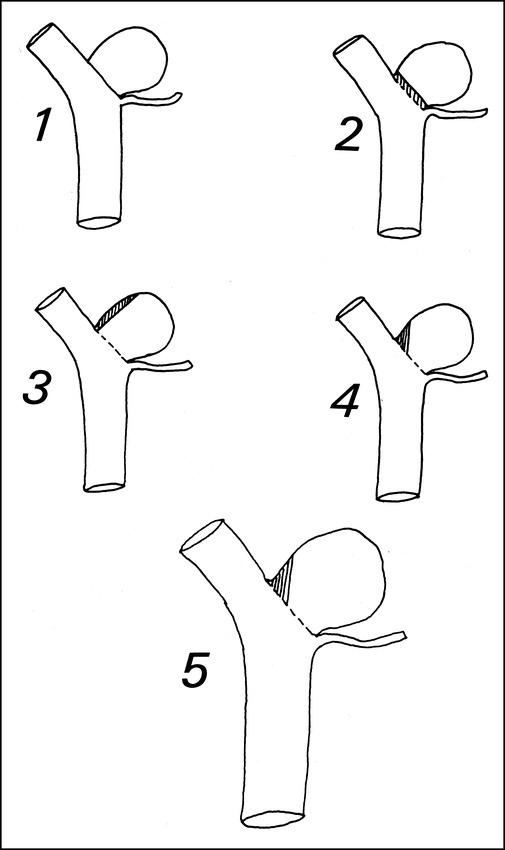
Illustration showing the sometimes poor correlation between percentage of occlusion and rest size (length of exposed aneurysmal wall after coiling). Aneurysms 1 through 4 are identical tracings. Aneurysm 1 has 100% occlusion and no rest. Aneurysms 2 through 4 all have 95% occlusion, but quite different rest sizes. The largest rest is in aneurysm 3 and the smallest in aneurysm 2. Aneurysm 5 is a magnified image of aneurysm 4, with the same percentage of occlusion but a larger rest size
The presence of angiographic vasospasm at the time of treatment was noted. When present, the linear reference value (15) was invalidated. Instead, the follow-up study (without vasospasm) was used to calculate the diameter of the midpoint of the petrous portion of the internal carotid artery. Since this portion is not affected by posthemorrhagic vasospasm, it will have the same absolute value in both the treatment and follow-up studies. For posterior circulation aneurysms with vasospasm (n = 3), the diagnostic angiograms (without vasospasm) were used to calculate linear measurements (15).
Other information collected included age, sex, time between treatment and follow-up, aneurysmal site (as internal carotid, anterior, or middle cerebral and vertebrobasilar), and clinical presentation (as mass effect, hemorrhage, or incidental aneurysm). Hemorrhages were classified as acute if treated within 15 days of ictus, and as delayed if 16 days or more had elapsed. Aneurysms were classified as sidewall, bifurcation or terminal, anterior communicating, and vertebrobasilar junction. The extent of luminal packing was subjectively noted on a scale of 0 to 6, with occlusion of the fundus, body, and neck of the aneurysm earning 0 to 2 units each (0 = no coils or a single loop, 1 = loose packing with contrast staining visible on angiography, and 2 = tight packing without visible contrast staining).
Success at treatment and at follow-up was defined as a rest size equal to or less than 2 mm. Changes in size of the residue (Figs 1 and 2), if any, between treatment and follow-up were defined as a difference of more than 2.5% occlusion or a 0.25-mm rest size (whichever was the smaller) and classified as 1) no change, 2) decrease or disappearance, 3) increase because of coil compaction, or 4) growth on the basis of expansion of the aneurysmal wall.
Success at treatment and at follow-up was analyzed using a χ2 test versus aneurysmal site, aneurysmal type, maximum dimension of the aneurysmal lumen (small, <10 mm; large, 10–25 mm; and giant, >25 mm), neck size to the nearest whole number, clinical presentation, presence of vasospasm, and degree of packing (Table 1). Changes in the aneurysmal residue were correlated against these variables (Table 1). The success rate for aneurysms treated acutely versus those treated more than 16 days after a hemorrhage was analyzed using the Fisher exact test (Table 2). No attempt was made to correlate clinical outcome with angiographic success or failure at the time of follow-up.
TABLE 1:
Influence of variables in the aneurysms versus success at time of treatment, success at follow-up, and change in the aneurysm
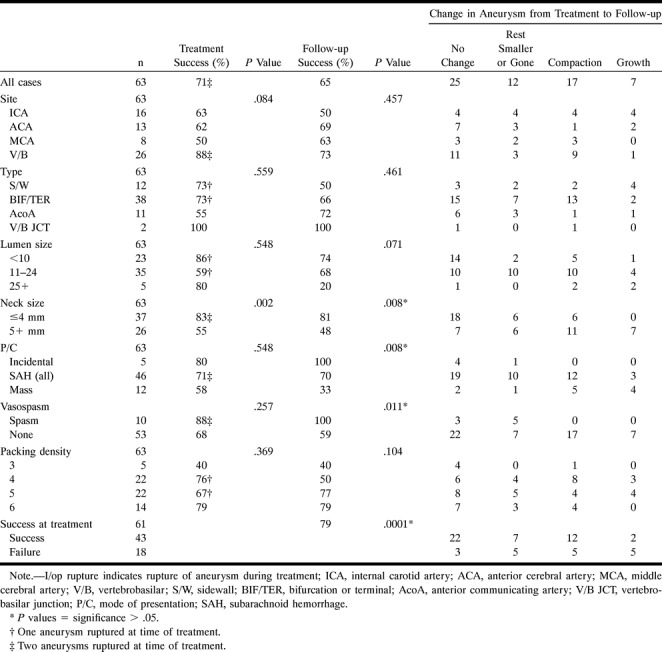
TABLE 2:
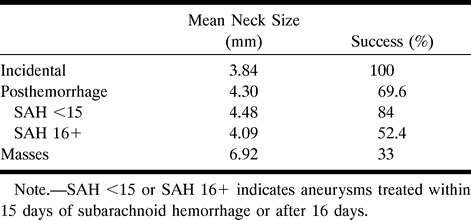
Mode of presentation by mean neck size and percentage of success at follow-up
Results
From a cohort of 74 patients treated between June 1992 and April 1995, 58 patients fulfilled the study entry criteria. Sixteen patients were excluded because either no follow-up angiogram was available or because the follow-up angiogram did not satisfy entry criteria. The 58 patients were treated for 63 aneurysms, and comprised 23 men and 35 women, with a mean age of 51 years (range, 18–73 years). Mean time to follow-up angiography was 7 months (range, 0.25–14 months), with 90% being more than 4 months.
Success, as defined by our criteria (rest size, <2 mm), was achieved in 43 (70%) of 61 aneurysms at treatment and in 41 (65%) of 63 aneurysms at follow-up. Aneurysmal occlusion, expressed as a percentage of the lumen, was, at completion of treatment (n = 61), less than 80% (n = 5), 80% to 90% (n = 7), greater than 90% but less than 100% (n = 32), and 100% (n = 17). At follow-up (n = 63), aneurysmal occlusion was less than 80% (n = 13), 80% to 90% (n = 11), greater than 90% but less than 100% (n = 17), and 100% (n = 22). The difference in the number of aneurysms between treatment (n = 61) and follow-up (n = 63) is accounted for by two intraprocedural ruptures that prevented measurement of percentage of occlusion at the time of treatment. These two were included in the analysis of success at follow-up.
The various factors that influenced success at the time of treatment and at follow-up, as well as their P values, are presented in Table 1. The frequency of change in the aneurysm between treatment and follow-up according to the various factors is also provided in Table 1. The only factor to achieve significance in predicting success at the time of treatment was neck size (P = .002, with 86% success for aneurysmal necks ≤4 mm and 50% success for aneurysmal necks ≥4 mm).
Factors that achieved significance in predicting success at the time of follow-up included neck size (P = .008), success at the time of treatment (P = .0001), and the presence of vasospasm at the time of treatment (P = .011). However, success at the time of treatment has already been indicated to be dependent on neck size, and a closer inspection of the cases with vasospasm indicates that this significance (P = .011) is also likely to be a reflection of neck size (mean neck sizes in aneurysms with and without vasospasm were 3.37 mm and 5.04 mm, respectively).
The mode of presentation of an aneurysm as incidental, posthemorrhagic, or as a mass appears to be a predictor of success at the time of follow-up (P = .008) (Table 1). Differences in mean neck size (3.84 mm, 4.30 mm, and 6.92 mm) can be used to explain the differences in success in the three main groupings of incidental (100%), posthemorrhagic (70%), and aneurysms with mass effect (33%), with success at follow-up being inversely proportional to neck size. In Table 2, the mode of presentation has been expanded, indicating that the success rate for aneurysms treated within 15 days of ictus (84%) is greater than for those treated after 16 days (52%) (P = .045, Fisher exact test), despite both a larger mean neck size (4.48 mm vs 4.09 mm) and a larger number of wide-necked aneurysms in the acutely treated group (11 of 25 vs five of 21).
A number of other observations were made that did not achieve statistical significance. The rate of success at the time of treatment was greatest in vertebrobasilar aneurysms (88%). At follow-up, the rate of success was greatest in basilar-tip (12 of 15) and anterior communicating artery (eight of 11) aneurysms, and lowest in internal carotid (eight of 16) and sidewall aneurysms (six of 12).
In 25 aneurysmal residues (41%) there was no change in the interval between treatment and follow-up. Twelve (20%) decreased in size or disappeared, 17 (28%) increased in size because of coil compaction, and seven (11%) sustained new growth. (In two aneurysms, no posttreatment recording was possible, thus change could not be determined.)
Thirty-six aneurysms (59%) had a measurable change between treatment and follow-up; however, this change was usually small and was only sufficient to alter its status as a success or failure in 14 aneurysms (23%). Of these 14, nine successes converted to failures and five failures converted to successes, for a net deficit of failures between treatment and follow-up of four aneurysms.
Aneurysmal growth occurred in seven cases and was seen most frequently in internal carotid (four of 16) and sidewall (four of 11) aneurysms, in initial treatment failures (five of 18), in large and giant aneurysms (six of 39), and in wide-necked aneurysms (seven of 26).
Compaction was least common in anterior communicating artery aneurysms (one of 11) and most common in vertebrobasilar aneurysms (nine of 24). Compaction was slightly more common in wide-necked (nine of 26) and poorly packed (nine of 26 for packing grades 3–4) aneurysms, and in those occurring as mass lesions (five of 12).
No change or partial/complete thrombosis of the aneurysmal residue (between treatment and follow-up) may be regarded as a stable residue. This stability was seen more frequently in anterior communicating (nine of 11) and narrow-necked (24 of 35) aneurysms and in the presence of vasospasm (eight of eight) and good packing (14 of 26 for grades 3–4 and 10 of 14 for grade 6). In descending order, the most stable embolizations were in incidental aneurysms (five of five), aneurysms treated acutely after hemorrhage (17 of 23), aneurysms treated 16 or more days after hemorrhage (12 of 21), and aneurysms occurring as mass lesions (three of 12).
By comparing the results from the first 31 aneurysms treated with the results from the next 32 aneurysms, a learning curve was determined: success at treatment and at follow-up was 61% and 52% for the first 31 aneurysms, and 80% and 78% for the second group. The second group also showed a higher percentage of stable rests (73% vs 52%) and a lower rate of occurrence of compaction (17% vs 36%). The two perforations that occurred were in the second group, reflecting our unit's move toward treating a larger number of ruptured aneurysms.
Discussion
Limitations of the Study
The study population was derived from patients with aneurysms who were referred for endovascular therapy at our unit between 1992 and 1995. Referral was based on anticipated surgical difficulty or surgical failure, and the aneurysms in the group of patients studied were generally representative of those aneurysms that were then treated by the endovascular route (1, 2, 4). The greater number of posterior circulation aneurysms and large and giant aneurysms in this study reflects the increased surgical morbidity in these patients (9, 18, 19). The study, therefore, is not representative of the population with aneurysms treated surgically (7, 20, 21), and caution should be exercised in extrapolating data derived from it to the general population. A second caveat is derived from the selection criteria that required preservation of the parent artery, thus excluding those aneurysms treated by endovascular parent artery occlusion. Finally, the aneurysms treated represented the early experience of the authors, and current success rates would be expected to be greater with more recently treated aneurysms because of improved selection criteria, treatment strategies, and the availability of a greater range of coil sizes and types. The higher success rates at treatment and at follow-up as well as the lower rate of occurrence of compaction in the second group of 32 aneurysms treated is illustrative of this point.
A limitation of the study is the lack of any correlation between angiographic success or failure and clinical outcome. At the time of the study, our unit was one of the few centers in Britain providing this treatment, and most referrals were from outside centers. Clinical records, therefore, were difficult to obtain within the period of data analysis and were incomplete as compared with the angiographic data. A decision was made to study only the angiographic data as well as any complete clinical data that were available at the time of referral.
The method used to measure the degree of packing of the aneurysmal lumen is a subjective one and, as such, may be open to criticism. The degree of packing was assessed as a factor in the success of treatment, because experimental studies had shown that compaction occurred after coil placement (13, 14). Graves et al (13) reported in a longitudinal study that compaction occurred in the first week after coil placement, and Mawad et al (14) reported that compaction was a cause of treatment failure. Although we noted a trend for more tightly packed aneurysms to have fewer treatment failures at follow-up (40%, 50%, 77%, and 79% for packing grades 3, 4, 5, and 6, respectively), this trend did not achieve statistical significance (Table 1). In part, this failure to establish significance may be a reflection of the subjective method used to measure the degree of packing.
The reference standard for linear measurements was derived from Zubillaga et al (15). While this decision may be criticized on the grounds that we have studied a different population, it does at least provide a “norm,” so that the same error, if any, applies to all cases. Second, this reference value cannot be applied in cases in which vasospasm is present (15). In those patients, a different method was used (see Methods). It may be argued that because seven of the 10 patients with vasospasm were in the acutely treated group, use of a different linear reference value in this group introduces a bias that might account for the higher success rate observed in the acutely treated group (84%) versus those treated after 16 days (52%) (Table 2). Exclusion of those patients with vasospasm from analysis, however, actually raises the mean neck size in the acutely treated group to 5.05 mm (4.13 mm for those with delayed treatment) and would only slightly affect the differential success in the two groups (77% for acutely treated patients and 47% for those with delayed treatment), so that use of a different reference value for linear measurements does not appear to be a potentially significant error.
A number of other potential sources of error arise out of the methodology. The first stems from the differences in magnification used when obtaining angiograms. These differences in magnification were corrected for by using a standard lecture room overhead projector to project the angiographic images onto the calibrated paper. By adjusting the distance from the projector to the wall on which the paper was pinned, different degrees of magnification were created that allowed the projected images to be accurately superimposed on one another.
Differences in the angle of projection between the different angiograms might have been more problematic were it not for the fact that the angle of projection that was used for the pretreatment and posttreatment studies was one of the two projections used in coil embolization. This same projection angle was used immediately before deployment of the first coil (the pretreatment angiogram) and at the end of the coiling (the posttreatment study) and was chosen because, among other factors, it best displayed the relationship of the aneurysm and its neck to the parent artery and its branches. A greater difficulty arose when matching these two projections with the follow-up angiogram obtained months later. While it was our practice at the time of follow-up to reproduce as closely as possible the same projection used during embolization, this was not always identical, as shown in Figures 1 and 2, particularly if this projection was not the one that showed compaction or growth to its greatest extent (Fig 1). In the latter situation, we would search the pretreatment and posttreatment angiograms to find the projection that most closely approximated the follow-up study. At all times in the study we attempted to match the anatomy at the neck region above all else in order to calculate changes in the rest as accurately as possible.
We defined a change in the aneurysmal residue between treatment and follow-up as having occurred when the percentage of occlusion changed by greater than 2.5% or when the rest had changed by greater than 0.25 mm. We minimized errors in this calculation by 1) using paper calibrated to 1 mm, 2) always maximally projecting the image of the aneurysm to cover the largest number of whole squares (usually between 200–400), 3) counting partial squares as well as whole ones, and 4) repetitively measuring images of coiled and uncoiled aneurysms before beginning the study until we could achieve an error rate less than the change we were searching for. We accept, however, that errors in our methodology will probably overestimate the number of aneurysms that changed between treatment and follow-up.
Finally, it is axiomatic that aneurysms are 3D structures that cannot be easily reduced to a 2D analysis. It is our hope that a future study that looks at changes in the aneurysm using a 3D analysis will confirm our findings.
In previous reports, the angiographic measurement of the success of endovascular treatment of aneurysms has usually been presented as a subjective percentage of occlusion (1–4, 15, 22) or simply as a descriptive term, such as “incompletely treated” (14). Objective measurements of the percentage of occlusion have the advantage of reproducibility and can be used, as in this study, to assess subtle changes in the residual lumen in longitudinal studies. This method, however, is time-consuming and contains two flaws. First, the same percentage of occlusion in different-sized aneurysms will produce different-sized residual lumens, and, second, the same percentage of occlusion in identically sized aneurysms can leave different lengths of the aneurysmal wall exposed (Fig 3). An alternative is to measure the size of the aneurysmal rest. This measurement of the rest size is quick, reproducible, and widely used in the neurosurgical literature (16, 17, 23–27) (allowing comparison between surgical and endovascular techniques) and establishes a correlation between rest size and risk of rehemorrhage (25). While the minimum size at which a rest is safe from rehemorrhage is controversial (17, 26), one apparently generally held view among various authors is that aneurysmal rests of less than 2 mm carry a basically benign prognosis (17). On this basis, we defined successful treatment as a rest size equal to or less than 2 mm.
Factors Influencing Success Rates
Because of the way we defined success, our results at follow-up will appear to be greater than that of others who defined success as 100% occlusion (4). Applying this same 100% criterion to our aneurysms, however, produces success rates that are almost identical.
By our definition of success, 35% of our aneurysms were failures at follow-up. Even allowing for the increased number of posterior circulation aneurysms in this series, with their higher rate of occurrence of rests (9, 17), this failure rate is significantly higher than the 5% observed in surgical series (16, 17). Even though angiographic follow-up of small surgical rests is not commonly associated with enlargement and rehemorrhage (16), nonetheless an increased risk of such an event exists in incompletely treated aneurysms (11, 23–26, 28–30), and it is important that the factors that influence success be studied to enable better case selection.
Previous studies have reported that luminal size, neck size, and, experimentally, the degree of packing of the aneurysmal lumen are factors that influence successful treatment of aneurysms (2–4, 13–15, 31). Hilal argued the importance of neck size (31), noting that smaller necks would be more likely to be bridged by the mesh of the coil ball, thus excluding the aneurysmal lumen from the parent circulation and allowing the endothelium to cover the neck. A success rate of 85% was achieved for aneurysms with necks 4 mm or less versus 16% for wide-necked aneurysms (15). Our results confirm the importance of neck size as a predictor of success, both at treatment (P = .002) and at follow-up (P = .008). The importance of neck size is reinforced by the finding that both vasospasm (P = .011) and success at the time of treatment (P = .0001) were predictors of success at follow-up. In addition, neck size influences the likelihood of change in the rest, both directly as well as indirectly, through its correlation with luminal diameter, vasospasm, and success at treatment.
Although we have confirmed the trend noted by others for luminal size to influence success (1–3), we do not believe that it is an independent predictor of success, and any significance of luminal size as a factor in this study (P = .07) is likely to be secondary to its direct correlation with neck size (mean neck sizes for small, medium, and large aneurysms were 3.35 mm, 5.34 mm, and 8.2 mm, respectively). We believe that this same argument holds true for the significance of luminal diameter as a factor influencing change in the rest.
The success or failure of treatment at follow-up, according to mode of presentation, is presented in Tables 1 and 2. Our results suggest a further significant differential success rate for the aneurysms treated before and more than 15 days after hemorrhage (84% and 52%, respectively) that cannot be explained on the basis of differences in mean neck size or number of wide-necked aneurysms between these two groups. This observation raises the intriguing possibility that the acutely ruptured aneurysm may be more receptive to thrombosis of the lumen after coil placement than those that are treated after an interval. Evidence that might support this suggestion comes from two sources. Casasco et al (2) treated 71 aneurysms with fiber coils, 67 of them after a hemorrhage, and noted a 100% occlusion rate in 85%. This is a significantly higher success rate than that recorded in this study or elsewhere (4) and may reflect a higher percentage of cases treated after hemorrhage. Part of this success, however, may be explained by the increased thrombogenicity of the fiber coils used (13). Also, endoluminal thrombus may transiently form within an aneurysmal lumen after rupture (32–34), and it is speculated that placement of a coil into the thrombotic milieu of a recently ruptured aneurysm may, with the additional electrothrombotic effect induced by coil detachment (22), encourage aneurysmal thrombosis. Our suggestion that early treatment may be associated with a higher success rate is based on a small number of cases and requires confirmation by a larger study.
Morphologic Changes in the Aneurysmal Residue
Changes in size of the aneurysmal rest between treatment and follow-up have been observed after both coiling and surgical clipping (2, 4, 16) and include decreases in size or obliteration of the residue as well as, less commonly, an increase in its size (16). In this study, changes were observed in 36 (59%) of 61 treated aneurysms. While this suggests that the aneurysmal residues were unstable, the criteria used to decide that a change had occurred were deliberately severe, so as to provide a large enough group for a meaningful analysis of change. In fact, the number of aneurysms that actually changed their status as successes or failures as a result of these changes was much smaller (14 [23%] of 61) and the net deficit of successes was even smaller (four [6.6%] of 61). An example of this is the apparently contradictory finding that while compaction was seen in nine (35%) of 26 vertebrobasilar aneurysms, the difference in success rates at treatment and follow-up was only 14%. The likelihood of change in rest morphologic features appears to depend primarily on neck size, with both growth and compaction occurring more frequently in wide-necked aneurysms. Growth of a preexisting aneurysm occurs from its wall (35), and, to prevent growth after coiling, successful treatment of the aneurysm must isolate the thinner wall of the aneurysm from the hemodynamic forces that contribute to its enlargement (6, 10, 36). Since rehemorrhage after surgical treatment correlates with the presence of a rest (5–11) and this risk increases with increasing rest size (25), it is predictable that aneurysmal growth after endovascular coiling would likewise occur more often in the presence of treatment failure, and particularly those failures that had larger rests. This argument is supported by the finding that five growths occurred in initial treatment failures (n = 18), with a mean rest size of 6.4 mm (range, 4.7–10.4 mm). Wide-necked aneurysms are also more likely to have compaction at follow-up, presumably because hemodynamic forces are able to act on a larger surface area of the coil ball at the neck of the aneurysm. While we did not establish a significant link between the degree of packing and success at treatment, there was a trend toward significance at follow-up (P = .104). Well-packed aneurysms were less likely to change and less likely to show coil-ball compaction than less well-packed lumens, reinforcing experimental suggestions that this is an important factor in the stability of the residue (13, 14).
Strother et al (36) have reported that the flow within experimental aneurysms is predictable and that it varies primarily according to the relationship of the aneurysm to its parent artery. While we did not specifically address this assertion, we did observe a number of findings that lead us to suggest that this is an important variable in the success of endovascular therapy. Aneurysmal growth was most common in sidewall aneurysms, and compaction occurred most frequently in bifurcation and termination types. This may be a reflection of the differences in inflow patterns at the ostia of the aneurysms. Anterior communicating artery aneurysms, which are not easily placed into one of the types described by Strother et al (36), rarely had compaction (one of 11) or growth (one of 11), with nine of 11 having no change or progressive thrombosis. In this case, it is possible that turbulence induced by colliding inflows from the opposing A1 segments acts to encourage the formation of thrombus in the presence of coils within the aneurysmal lumen. Finally, compaction or growth of aneurysms was not seen with aneurysms treated in the presence of vasospasm, and five of eight such aneurysms had progressive thrombosis between treatment and follow-up. While this could be a reflection of the smaller neck size in those aneurysms with vasospasm, alterations in inflow hemodynamics occurring as a consequence of the vasospasm may be a contributing factor.
Footnotes
Address reprint requests to Dr. J. K. Ayton Hope, Auckland Public Hospital, Auckland, New Zealand.
References
- 1.Byrne JV, Adams CBT, Kerr RSC, et al. Endovascular treatment of inoperable intracranial aneurysms with platinum coils. Br J Neurosurg 1995;9:585-592 [DOI] [PubMed] [Google Scholar]
- 2.Casasco AE, Aymard A, Gobin YP, et al. Selective endovascular treatment of 71 intracranial aneurysms with platinum coils. J Neurosurg 1993;79:3-10 [DOI] [PubMed] [Google Scholar]
- 3.Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach, part 2: preliminary clinical experience. J Neurosurg 1991;75:8-14 [DOI] [PubMed] [Google Scholar]
- 4.Guglielmi G, Vinuela F, Duckwiler G, et al. Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coil. J Neurosurg 1992;77:515-524 [DOI] [PubMed] [Google Scholar]
- 5.Chyatte D. The epidemiology, genetics, and clinical behavior of intracranial aneurysms. In: Awad IA, ed. Current Management of Cerebral Aneurysms. Neurosurgical Topics Series, bk 15. Park Ridge, IL: American Association of Neurological Surgeons; 1993;1-20
- 6.Ferguson GG. Physical factors in the initiation, growth, and rupture of human intracranial saccular aneurysms. J Neurosurg 1972;37:666-677 [DOI] [PubMed] [Google Scholar]
- 7.Kassell NF, Torner JC, Jane AJ, et al. The International Cooperative Study on the Timing of Aneurysm Surgery, part 2: surgical results. J Neurosurg 1990;73:37-47 [DOI] [PubMed] [Google Scholar]
- 8.McKissock W, Richardson A, Walsh L. Anterior communicating aneurysms: a trial of conservative and surgical treatment. Lancet 1965;1:873-876 [PubMed] [Google Scholar]
- 9.Peerless SJ, Hernesniemi JA, Gutnam FB, Drake CG. Early surgery for ruptured vertebrobasilar aneurysms. J Neurosurg 1994;80:643-649 [DOI] [PubMed] [Google Scholar]
- 10.Stehbens WE. Etiology of intracranial berry aneurysms. J Neurosurg 1989;70:823-831 [DOI] [PubMed] [Google Scholar]
- 11.Todd NV. Aneurysm re-bleeding after treatments that leave the aneurysm sac patent. Br J Neurosurg 1990;4:373-379 [DOI] [PubMed] [Google Scholar]
- 12.Higashida RT, Halbach Van V, Barnwell SL, et al. Treatment of intracranial aneurysms with preservation of the parent vessel: results of percutaneous balloon embolization in 84 patients. AJNR Am J Neuroradiol 1990;11:633-640 [PMC free article] [PubMed] [Google Scholar]
- 13.Graves VB, Strother CM, Rappe AH. Treatment of experimental canine carotid aneurysms with platinum coils. AJNR Am J Neuroradiol 1993;14:787-793 [PMC free article] [PubMed] [Google Scholar]
- 14.Mawad ME, Mawad JK, Cartwright J, et al. Long-term histopathological changes in canine aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1995;16:7-13 [PMC free article] [PubMed] [Google Scholar]
- 15.Zubillaga AF, Guglielmi G, Vinuela F, et al. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol 1994;15:815-882 [PMC free article] [PubMed] [Google Scholar]
- 16.Feuerberg I, Lindquist C, Lindqvist M, et al. Natural history of postoperative aneurysm rests. J Neurosurg 1987;66:30-34 [DOI] [PubMed] [Google Scholar]
- 17.Mennonna P, Ammannati F, Bordi L, et al. Aneurysm rests in a surgical series of 275 patients (five years): incidence, prognostic and therapeutic implications. In: Pasqualin A, Da Pian R, eds. New Trends in Management of Cerebrovascular Malformations. New York: Springer; 1994;310-315
- 18.Battaglia R, Pasqualin A, Da Pian R. Italian cooperative study on giant intracranial aneurysms, I: study design and clinical data. Acta Neurochir Suppl (Wien) 1988;42:49-52 [DOI] [PubMed] [Google Scholar]
- 19.Pasqualin A, Battaglia R, Scienza R, et al. Italian cooperative study on giant intracranial aneurysms, IV: results of treatment. Acta Neurochir Suppl (Wien) 1988;42:65-70 [DOI] [PubMed] [Google Scholar]
- 20.Kassell NF, Torner JC, Haley EC, et al. The International Cooperative Study on the Timing of Aneurysm Surgery, part 1: overall management results. J Neurosurg 1990;73:18-36 [DOI] [PubMed] [Google Scholar]
- 21.Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations: based on 6368 cases in the cooperative study. J Neurosurg 1966;25:219-239 [DOI] [PubMed] [Google Scholar]
- 22.Guglielmi G, Vinuela F, Sepetka I, et al. Electrothrombosis of saccular aneurysms via endovascular approach. J Neurosurg 1991;75:1-7 [DOI] [PubMed] [Google Scholar]
- 23.Allcock JM, Drake CG. Postoperative angiography in cases of ruptured intracranial aneurysm. J Neurosurg 1963;20:752-759 [DOI] [PubMed] [Google Scholar]
- 24.Drake CG, Friedman AH, Peerless SJ. Failed aneurysm surgery. J Neurosurg 1984;61:848-856 [DOI] [PubMed] [Google Scholar]
- 25.Drake CG, Vanderlinden RG. The late consequences of incomplete surgical treatment of cerebral aneurysms. Neurosurgery 1967;27:226-238 [DOI] [PubMed] [Google Scholar]
- 26.Lin T, Fox AJ, Drake CG. Regrowth of aneurysm sacs from residual neck following aneurysm clipping. J Neurosurg 1989;70:556-560 [DOI] [PubMed] [Google Scholar]
- 27.Takeda M, Yonekawa Y, Miyake H, et al. Recurrent subarachnoid hemorrhage after aneurysm surgery. In: Pasqualin A, Da Pian R, eds. New Trends in Management of Cerebrovascular Malformations. New York: Springer; 1994;316-320
- 28.Drake CG, Allcock JM. Postoperative angiography and the “slipped” clip. J Neurosurg 1973;39:683-689 [DOI] [PubMed] [Google Scholar]
- 29.Sakaki T, Takeshima T, Tominaga M, et al. Recurrence of ICA-PcoA aneurysms after neck clipping. J Neurosurg 1994;80:58-63 [DOI] [PubMed] [Google Scholar]
- 30.Weir BKA, Drake CG. Rapid growth of residual aneurysmal neck during pregnancy. J Neurosurg 1991;75:780-782 [DOI] [PubMed] [Google Scholar]
- 31.Hilal SK. Endovascular treatment of aneurysms with coils (letter). J Neurosurg 1992;76:337-339 [DOI] [PubMed] [Google Scholar]
- 32.Iwanaga H, Wakai S, Ochiai C, et al. Ruptured cerebral aneurysms missed by initial angiographic study. Neurosurgery 1990;27:45-51 [DOI] [PubMed] [Google Scholar]
- 33.Kassell NF, Torner JC. Size of intracranial aneurysms. Neurosurgery 1983;12:291-297 [DOI] [PubMed] [Google Scholar]
- 34.Sengupta RP, McAllister VL. In: Subarachnoid Haemorrhage.New York: Springer; 1986;143
- 35.Suzuki J, Ohara H. Clinicopathological study of cerebral aneurysms: origin, rupture, repair, and growth. J Neurosurg 1978;48:505-514 [DOI] [PubMed] [Google Scholar]
- 36.Strother CM, Graves VB, Rappe A. Aneurysm hemodynamics: an experimental study. AJNR Am J Neuroradiol 1992;13:1089-1095 [PMC free article] [PubMed] [Google Scholar]


