Abstract
BACKGROUND AND PURPOSE: Magnetization transfer imaging provides information about the structural integrity of macromolecular substances, such as myelin. Our objective was to use this imaging technique and contour plotting to characterize and to define the extent of white matter lesions in multiple sclerosis and traumatic brain injury.
METHODS: Magnetization transfer imaging was performed of 30 multiple sclerosis plaques and 10 traumatic white matter lesions. Magnetization transfer ratios (MTRs) were calculated for the lesions, for the normal- or abnormal-appearing surrounding white matter, and for remote normal-appearing white matter. MTR contour plots were constructed about these lesions.
RESULTS: The contour plot appearance of MS plaques differed from that of traumatic white matter lesions. There was a gradual increase in MTR values at points at increasing distances from the center of the MS plaques; this was true for those lesions with and without surrounding T2 signal abnormality (halos). In contrast, there was an abrupt transition in MTR values between traumatic lesions and normal-appearing surrounding white matter. Additionally, the size of the MTR abnormality exceeded the size of the T2 signal abnormality for the MS plaques.
CONCLUSION: MTR contour plots permit characterization and border definition of white matter lesions. Analysis of the contour plots suggests that MS is a centrifugal process with the lowest MTR within the center of the lesion. In contrast, traumatic white matter injuries are discrete lesions with abrupt transitions between the abnormal lesion and normal brain.
Magnetization transfer imaging has been applied to the study of multiple sclerosis (MS) (1, 2), progressive multifocal leukoencephalopathy (3), amyotrophic lateral sclerosis (4), wallerian degeneration (5), traumatic brain injury (6), and metastatic disease (7). In many of these conditions, this technique has revealed abnormalities in white matter that have appeared normal on T2-weighted images. Magnetization transfer imaging is based on the principal that protons bound in macromolecular structures exhibit T1 relaxation coupling with protons in the aqueous phase. An off-resonance saturation pulse can be applied to selectively saturate the bound protons. Subsequent exchange of longitudinal magnetization with the free water protons leads to a resultant reduction in signal intensity detected from these free protons (8, 9). Thus, intact macromolecular structures with a relatively larger number of bound protons will exhibit relatively larger magnetization transfer effects, manifest by reductions in signal intensity in the presence of the saturation pulse. The magnetization transfer ratio (MTR) is defined by the equation MTR = (M0−Ms)/M0, where M0 = average signal intensity in the absence of the saturation pulse and Ms = average signal intensity in its presence. This value is technique-dependent but does provide a reproducible index of magnetization transfer effects and may be viewed as a quantitative measure of the structural integrity of tissues (10, 11).
MS has been estimated to affect as many as 350,000 young and middle-aged Americans, causing significant functional impairment (12). While MR imaging has proved useful in the diagnosis of MS, the association between lesion volume and clinical disability remains a controversial issue. This controversy may in part be attributed to the difficulty in quantitating lesion volume, as the identification of lesion borders on T2-weighted images by visual inspection is a subjective assessment (13–16). Histologic studies have revealed extensive abnormality within the white matter of patients with MS (17, 18), and previous studies using magnetization transfer imaging have found abnormally low MTRs in normal-appearing white matter in these patients (1, 2).
Traumatic brain injury is among the most common causes of neurologic morbidity and mortality in this country; the annual incidence of closed head injury has been estimated at 130 to 150 per 100,000 persons (19, 20). In the United States, approximately 373,000 victims of head trauma require hospitalization each year (21), and the rehabilitation of disabled survivors requires vast human and economic resources (22, 23). As in MS, the imaging appearance of brain injuries has not always corresponded to the patient's disability (24).
We developed a new method to interrogate individual white matter lesions, using magnetization transfer contour plots, thus allowing a statistical measure to be used to characterize lesions in individual patients. This was done in an effort to better delineate the extent of white matter abnormality and to potentially distinguish differences in white matter disease in various conditions. We subsequently applied these contour plots to a spectrum of lesions with very different pathogeneses; namely, to traumatic white matter lesions and to the inflammatory lesions of MS.
Subjects and Methods
MR images from 37 patients with the clinical diagnosis of MS and from eight patients with traumatic brain injury were reviewed. Images were acquired at 1.5 T, and imaging protocols included sagittal T1-weighted sequences and axial T2- and proton density-weighted sequences. In the patients with MS, axial contrast-enhanced T1-weighted images were also obtained. In the head injured patients, an axial T2*-weighted gradient-echo sequence was performed in order to improve detection of hemorrhage. Clinical data were obtained from retrospective chart review.
Magnetization transfer imaging was performed in both groups (before contrast administration) with a 3D gradient-recalled acquisition in the steady state (GRASS) sequence with parameters of 100/5 (TR/TE) and a flip angle of 12°. One 19-millisecond sinc-shaped off-resonance saturation pulse with a magnitude of 3.67 × 10−6T was used per TR; control images were obtained without the saturation pulse. The parameters used were chosen to minimize T1 and T2 weighting. Images were processed on a Sun SparcStation 330, equipped with IDL software (Interactive Data Language, Research Systems, Inc, Boulder, CO). Contour plots were subsequently applied to lesions identified in 10 of the MS patients and in four of the trauma patients. The 10 patients with MS included seven women and three men; their ages ranged from 32 to 44 years. Seven patients had relapsing-remitting MS and three had progressive disease. Imaging was performed during acute exacerbations in four of the patients. The four head-injured patients included three men and one woman, ages 19 to 33 years. Three were victims of motor vehicle accidents and one had suffered injuries in an industrial accident; all were initially imaged within 2 weeks of presentation. Normative data were obtained from images acquired in seven healthy volunteers, four women and three men (ages 20–38 years).
T2-weighted images were initially evaluated by a neuroradiologist. White matter lesions were regarded as either well circumscribed or as surrounded by an area of less pronounced hyperintensity, which we termed a halo (Fig 1). In those lesions with halos, MTRs were calculated for normal-appearing white matter, for halos, and for the center of the lesion; in the lesions that did not possess halos, MTRs were obtained in their center, in the surrounding white matter, and in normal-appearing white matter, remote from the lesions.
fig 1.
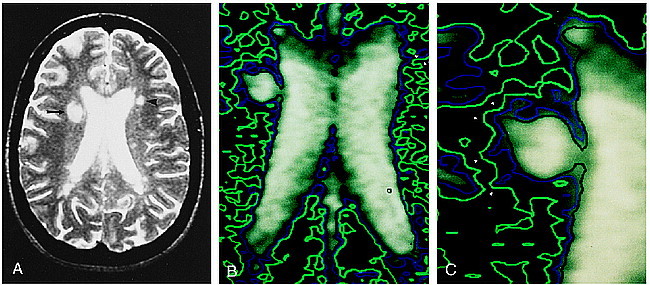
A, Axial T2-weighted (3000/90/1) image in a patient with MS. Right periventricular lesion is poorly defined, with a halo of signal abnormality (arrow). In contrast, left periventricular lesion appears well circumscribed (arrowhead).
B, Contour plot applied to magnetization transfer map. Regions in green delineate areas with MTR less than 0.39; those in blue delineate areas with MTR less than 0.35, and those in black depict areas with MTR less than 0.32. This contour map reveals that both periventricular lesions are actually poorly defined, with gradations of surrounding MTR abnormality. The center of the left periventricular lesion appears as a small focus surrounded by a black contour line (arrow). This is surrounded by a blue contour (arrowhead), which in turn is encompassed by a green contour (double arrowheads). In fact, the green contour line surrounds all of the periventricular white matter, confirming a diffuse abnormality of the magnetization transfer parameters of the white matter.
C, Magnified view of contour plot applied to an MS plaque reveals fingerlike projections of abnormally low MTR values (arrowheads) extending into the white matter surrounding the lesion.
Magnetization transfer images were overlaid with MTR contour plots, delineating regions with MTRs of less than 0.39, less than 0.35, and less than 0.32. The outermost contour (MTR less than 0.39) was selected to encompass regions that were statistically abnormal; MTRs in these areas were at least 2 standard deviations below the MTRs obtained in the white matter of healthy control subjects. Previous work has demonstrated that lesions that were only visible on T2-weighted images had an average MTR of less than 0.35, and lesions visible on T1-weighted images as well (presumably reflecting progressive myelin loss, axonal loss, and/or gliosis) had an average MTR of less than 0.32 (25). Inner contours were chosen to reflect these empirical parameters.
Results
Sixteen lesions with halos on T2-weighted images were identified in 10 of the 37 patients with MS. Fourteen lesions (in the same 10 patients) without apparent halos were also evaluated with this technique. Fourteen of the lesions with halos had ring/peripheral enhancement after contrast administration. Four of the eight patients with head injuries had 10 white matter lesions that were consistent with axonal injury and that were investigated with the contour plotting technique. Two of these lesions were surrounded by areas of hyperintensity on T2-weighted images.
MTRs obtained in white matter in various locations of the brains of seven healthy volunteers ranged from 0.42 ± 0.014 to 0.45 ± 0.023, with an overall average MTR value of 0.43 ± 0.016. Average MTRs within lesions with detectable halos seen in patients with MS are presented in Table 1. Similarly, a gradation of abnormal MTRs was seen radiating from the centers of lesions without obvious halos (Table 1). Figure 1A shows a periventricular lesion with a surrounding halo on the right; an apparently well-circumscribed lesion is present on the left. Figure 1B, the corresponding magnetization transfer map overlaid with a contour plot, reveals a gradation in magnetization transfer values about both lesions (black = 0.32, blue = 0.35, green = 0.39). Additionally, the borders of the lesions are poorly defined, with fingerlike projections of magnetization transfer abnormality extending into the surrounding white matter. These fingerlike projections of magnetization transfer abnormality are also depicted in Figure 1C.
Table 1:
Average MTR values in patients with MS

The average MTRs of lesions in the patients with traumatic brain injuries are presented in Table 2. The average MTR (0.43 ± 0.030) in the tissue surrounding these apparently well-circumscribed lesions was similar to that of normal white matter. In white matter remote from the lesions, the average MTR was 0.45 ± 0.019. In contrast to the findings seen with the lesions of MS, there was an abrupt transition in MTR values between the lesions and the normal-appearing surrounding white matter. Contour plots applied to these lesions also confirmed that the lesions were well circumscribed (Fig 2).
Table 2:
Average MTR values in patients with traumatic brain injury

fig 2.
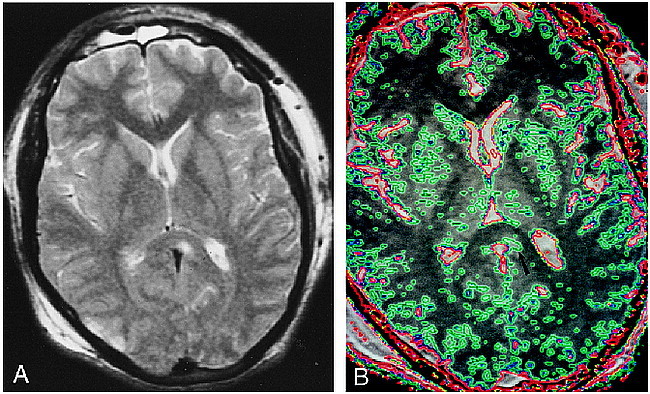
A, Axial T2-weighted (2500/90/1) image depicts a well-circumscribed nonhemorrhagic lesion in the splenium of the corpus callosum. There is also a small contusion in the right occipital cortex.
B, Contour plot applied to magnetization transfer map. Region of MTR abnormality is also shown to be well circumscribed and does not exceed the boundaries of the signal abnormality seen on the T2-weighted image (arrow). Area encircled with green depicts region with MTR less than 0.39 (blue = 0.35; red = 0.32). The MTR associated with normal gray matter is lower than that associated with normal white matter. The blue and green contours seen in the cortex, the basal ganglia, and the thalami do not represent areas of abnormality.
Discussion
In the white matter of patients with MS, an analysis of MTRs reveals a gradual increase in these values as white matter at progressively increasing distances from the center of the lesion is interrogated. A similar finding has been reported with metastatic lesions (7). Areas of abnormally decreased MTR are readily detected through the use of magnetization transfer contour plots. In disease processes such as MS, the region of magnetization transfer abnormality is often larger than the area of increased signal intensity seen on corresponding T2-weighted images. Thus, MTR contour plots suggest that MS lesions are more extensive than the signal abnormality detected by conventional MR imaging and that they extend into regions of normal-appearing white matter. Histologic inspection has also shown MS plaques to have poorly defined borders (26). In contrast, magnetization transfer contour plots applied to traumatic white matter injuries suggest that they are actually well circumscribed. The histopathologic findings of diffuse axonal injury include discrete axonal disruptions, foci of axonal swelling and retraction bulbs, and microglial proliferation, with or without associated focal hemorrhage (22, 27–31). Hence, the contour plot appearance of traumatic white matter lesions corresponds to their histologic appearance and contrasts with the appearance of MS plaques.
Contour plot analysis can also aid in the characterization of white matter lesions. Lower MTRs, such as those seen within lesions that are hypointense on T1-weighted images, have been thought to indicate a greater degree of demyelination and axonal loss (18, 25). Multilevel contour plots can display areas that have undergone such demyelination or axonal loss and potentially differentiate them from otherwise abnormal white matter. The average MTR values obtained in the center of traumatic lesions were slightly higher than those obtained in MS plaques. This was a somewhat unexpected finding, as lesions with complete axonal loss would presumably be associated with the lowest MTRs. A multitude of factors most likely contributed to this result. A spectrum of traumatic lesions, both hemorrhagic and bland, with MTRs ranging from 0.10 to 0.39, were imaged. Additionally, three of the four trauma patients were imaged acutely or subacutely (at 5 to 11 days after injury), when processes such as wallerian degeneration and demyelination may have been ongoing. Last, Trapp et al (18) have recently shown that axonal transection is a frequent finding in MS plaques.
The application of multilevel contour plots may also aid in differentiating otherwise nonspecific white matter lesions. As stated above, the contour plot appearance of traumatic lesions differs from that of the inflammatory lesions of MS, particularly with respect to the surrounding white matter. Further investigation into the potentially unique contour plot appearance of ischemic and infectious white matter lesions is warranted.
Prior studies have yielded controversial results regarding correlation of lesion volume and clinical disability. However, identification of lesion borders on T2-weighted images is a subjective assessment; hence, quantitation of lesion volume is itself a problematic issue (13–16). Additionally, lesion location also has a likely impact on symptoms and disability. Prior attempts at quantification of disease burden in MS have included manual and automated counting of lesions and histogram analysis of 3D magnetization transfer data. Contour plot applications may also aid in white matter lesion quantification. The studies of van Buchem et al (32–34) have suggested a correlation between MTR histogram peak height, an estimate of global disease burden, and neurologic impairment and chronicity of disease. Like MTR histogram analysis, contour plot applications are automated, objective, rapid (requiring a few seconds for each section being evaluated), and capable of revealing normal-appearing white matter with abnormally reduced MTRs. Contour plots use a statistical measure to define lesion borders, which often extend beyond the regions of T2 signal abnormality and thus may provide a better estimate of disease burden than does visual inspection of images. These global assessments of disease burden may be used to monitor disease course and response to therapy.
Conclusion
Contour plot applications provide a rapid, automated, and reproducible technique for the detection of abnormal white matter, as judged by an objective measure, the MTR. With this technique, T2-occult lesions may be detected, and lesion borders may be more precisely defined. Initial results have shown considerable differences in the contour plot appearances of traumatic white matter lesions and MS plaques. Such results give promise for improved characterization of additional nonspecific white matter abnormalities.
Acknowledgments
We thank Frank Lexa for his assistance with the normative data and Lois Mannon for her assistance in gathering clinical data.
Footnotes
Supported by NIH NS34353, NINDS-NS08803, and RSNA Research and Education Fund.
Address reprint requests to Linda J. Bagley, MD, Department of Radiology, University of Pennsylvania Medical Center, 3400 Spruce St, Philadelphia, PA 19104.
References
- 1.Loevner LA, Grossman RI, Lexa FJ, Kessler D. Microscopic disease in normal-appearing white matter on conventional MRI in patients with multiple sclerosis: assessment with utilizing magnetization transfer measurements. Radiology 1995;196:511-515 [DOI] [PubMed] [Google Scholar]
- 2.Filippi M, Campi A, Dousset V, et al. A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology 1995;45:478-482 [DOI] [PubMed] [Google Scholar]
- 3.Kasner SE, Galetta SL, McGowan JC, Grossman RI. Magnetization transfer imaging in progressive multifocal leukoencephalopathy. Neurology 1997;48:534-536 [DOI] [PubMed] [Google Scholar]
- 4.Kato Y, Matsumura K, Kinosada Y, Narita Y, Kuzuhara S, Nakagawa T. Detection of pyramidal tract lesions in amyotrophic lateral sclerosis with magnetization transfer measurements. AJNR Am J Neuroradiol 1997;18:1541-1547 [PMC free article] [PubMed] [Google Scholar]
- 5.Lexa FJ, Grossman RI, Rosenquist AC. Wallerian degeneration in feline visual system: characterization with magnetization rate with histopathological correlation. AJNR Am J Neuroradiol 1994;15:201-212 [PMC free article] [PubMed] [Google Scholar]
- 6.Bagley LJ, Grossman RI, McGowan JC, Sinson GP. Magnetization transfer imaging in the detection of T2-occult white matter lesions: a predictor of outcome in traumatic brain injury? Presented at the International Society of Magnetic Resonance in Medicine, Vancouver, 1997
- 7.Boorstein J, Wong KIT, Grossman RI, Bologna L, McGowan JC. Metastatic lesions of the brain: imaging with magnetization transfer. Radiology 1994;191:799-803 [DOI] [PubMed] [Google Scholar]
- 8.Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 1989;10:135-144 [DOI] [PubMed] [Google Scholar]
- 9.Wolff SD, Eng J, Balaban RS. Magnetization transfer contrast: method for improving contrast in gradient-recalled-echo images. Radiology 1991;179:133-137 [DOI] [PubMed] [Google Scholar]
- 10.Dousset V, Grossman RI, Ramer KN, et al. Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology 1992;182:483-491 [DOI] [PubMed] [Google Scholar]
- 11.McGowan JC, Schnall MD, Leigh JS. Magnetization transfer imaging with pulsed off-resonance saturation: variation in contrast with saturation duty cycle. J Magn Reson 1994;4:79-82 [DOI] [PubMed] [Google Scholar]
- 12.Anderson DW, Ellenber JH, Leventhal CM, et al. Revised estimate of the prevalence of multiple sclerosis in the United States. Ann Neurol 1992;31:333-336 [DOI] [PubMed] [Google Scholar]
- 13.Huber SJ, Paulson GW, Chakeres D, et al. Magnetic resonance imaging and clinical correlations in multiple sclerosis. J Neurol Sci 1988;86:1-12 [DOI] [PubMed] [Google Scholar]
- 14.Truyen L, Gheuens J, et al. Improved correlation of magnetic resonance imaging (MRI) with clinical status in multiple sclerosis (MS) by use of an extensive standardized imaging protocol. J Neurol Sci 1990;96:173-182 [DOI] [PubMed] [Google Scholar]
- 15.Baumhefner RW, Tourtellotte WW, Syndulko K, et al. Quantitative multiple sclerosis plaque assessment with magnetic resonance imaging: its correlation with clinical parameters, evoked potentials, and intra-blood-brain barrier IgG synthesis. Arch Neurol 1990;47:19-26 [DOI] [PubMed] [Google Scholar]
- 16.Thompson AJ, Kermode AG, MacManus DG, et al. Patterns of disease activity in multiple sclerosis: clinical and magnetic resonance imaging study. BMJ 1990;600:631-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen I, McKeown S. A histological, histochemical and biochemical study of macroscopically normal white matter in multiple sclerosis. J Neurol Sci 1979;41:81-91 [DOI] [PubMed] [Google Scholar]
- 18.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278-285 [DOI] [PubMed] [Google Scholar]
- 19.Kraus JF, Black MA, Hessol N, et al. The incidence of acute brain injury and serious impairment in a defined population. Am J Epidemiol 1984;119:186-201 [DOI] [PubMed] [Google Scholar]
- 20.Kraus JF, Nourjah P. The epidemiology of mild, uncomplicated brain injury. J Trauma 1988;28:1637-1643 [DOI] [PubMed] [Google Scholar]
- 21.Collins JG. Types of injuries by selected characteristics: United States. Vital Health Stat 1990;10:175. [PubMed] [Google Scholar]
- 22.Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 1995;45:1253-1260 [DOI] [PubMed] [Google Scholar]
- 23.Farber M. Head injuries: the worst case. Sports Illustrated 1994;81:39-53 [Google Scholar]
- 24.Wilberger JE, Rothfus WE, Tabas J, et al. Acute tissue tear hemorrhages of the brain: computed tomography and clinicopathological correlations. Neurosurgery 1990;27:208-213 [DOI] [PubMed] [Google Scholar]
- 25.Loevner LA, Grossman RI, McGowan JC, Ramer KN, Cohen JA. Characterization of multiple sclerosis plaques with T1-weighted MR and quantitative magnetization transfer. AJNR Am J Neuroradiol 1995;16:1473-1479 [PMC free article] [PubMed] [Google Scholar]
- 26.Allen IV. Pathology of multiple sclerosis. In: Matthews WB, ed. McAlpine's Multiple Sclerosis. 2nd ed. Edinburgh: Churchill Livingstone; 1991:341–378
- 27.Adams JH, Doyle D, Ford I, Gennarelli TA. Diffuse axonal injury and head injury: definition diagnosis and grading. Histopathology 1989;15:49-59 [DOI] [PubMed] [Google Scholar]
- 28.Clark EJM. Distribution of microglial clusters in the brain after head injury. J Neurol Neurosurg Psychiatry 1974;37:463-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crooks DA. A method to quantitate axonal injury. Neuropathol Appl Neurobiol 1991;17:421-424 [DOI] [PubMed] [Google Scholar]
- 30.Oppenheimer DR. Microscopic lesions in the brain following head injury. J Neurol Neurosurg Psychiatry 1968;31:299-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Povlishock JT, Becker DP, Cheng CLY, Vaughan CW. Axonal change in minor head injuries. J Neuropathol Exp Neurol 1983;42:225-242 [DOI] [PubMed] [Google Scholar]
- 32.van Buchem MA, McGowan JC, Kolson D, Polansky M, Grossman RI. Quantitative volumetric magnetization transfer analysis in multiple sclerosis: estimation of macroscopic and microscopic disease burden. Magn Reson Med 1996;36:632-636 [DOI] [PubMed] [Google Scholar]
- 33.van Buchem MA, Udupa JK, McGowan JC, et al. Global volumetric estimation of disease burden in multiple sclerosis based on magnetization transfer imaging. AJNR Am J Neuroradiol 1997;18:1287-1290 [PMC free article] [PubMed] [Google Scholar]
- 34.van Buchem MA, Grossman RI, Armstrong C, et al. Correlation of quantitative volumetric magnetization transfer imaging with clinical and neuropsychological data in multiple sclerosis. Neurology 1998;50:1609-1617 [DOI] [PubMed] [Google Scholar]


