Abstract
Summary: We report a unique case of metastatic craniopharyngioma. Initially, the patient had a right frontal craniotomy for resection of a suprasellar mass, which was determined to be an adamantinomatous craniopharyngioma. Seven years later, an MR study of the brain showed two peripheral enhancing lesions adjacent to the dura and contralateral to the craniotomy site. Pathologic examination again showed adamantinomatous craniopharyngioma. Although recurrence, both local and along surgical tracts due to implantation of craniopharyngioma tissue, has been reported, this case raises the possibility of meningeal seeding to remote sites.
Craniopharyngiomas are complex epithelial tumors arising from remnants of Rathke's pouch (1, 2). Total resection of a craniopharyngioma may be difficult, and recurrence has been reported in approximately 25% to 70% of patients (3, 4). Recurrence usually occurs at the primary site, although a few cases of ectopic recurrence along the surgical or needle tracts have been reported (5–7). We present a case of metastatic craniopharyngioma that cannot be attributed to local recurrence or to direct implantation by the surgical procedure. To our knowledge, no other cases of metastatic craniopharyngioma have been reported to date.
Case Report
A 73-year-old man initially presented in March 1990 with symptoms of bitemporal hemianopsia. An MR study of the brain (Fig 1A) showed a 2-cm complex partially enhancing suprasellar mass, which elevated and stretched the optic chiasm and compressed the floor of the third ventricle. This mass had a cystic-appearing area of low signal intensity on T1-weighted images and high signal intensity on T2-weighted images, with a small, enhancing mural nodule. No other areas of abnormality were detected at this time. The lesion was surgically excised through a right frontal craniotomy, and pathologic examination revealed adamantinomatous craniopharyngioma.
fig 1.
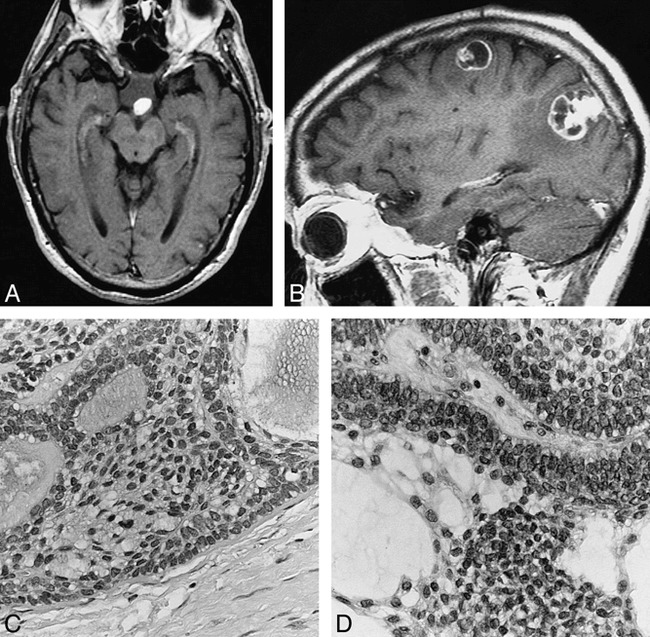
73-year-old man with metastatic craniopharyngioma.
A, In 1990, axial contrast-enhanced T1-weighted MR image (800/20 [TR/TE]) of the brain shows a 2-cm suprasellar mass with a solidly enhancing nodule along its posterior margin.
B, In 1997, left parasagittal contrast-enhanced T1-weighted MR image (540/14) of the brain shows two dural-based lobulated enhancing lesions. The left parietooccipital convexity lesion has a 2-cm cystic-appearing component attached to an enhancing dural-based pedicle. The left frontal lesion is smaller and has irregular mixed solid and rim enhancement characteristics.
C, In 1997, histologic section of the left parietooccipital lesion is composed of solid and cystic epithelial nests with distinct peripheral palisading and central loosely cohesive cells termed stellate reticulum (hematoxylin-eosin, original magnification ×400).
D, In 1990, the original suprasellar mass contains identical epithelial nests with peripheral palisading and central stellate reticulum (hematoxylin-eosin, original magnification ×400).
In October 1997, the patient presented with a new onset of partial seizures. An MR examination of the brain (Fig 1B) showed two peripheral enhancing lesions adjacent to the dura, in the left parietooccipital and posterior left frontal convexities. There was no recurrent or residual suprasellar mass. At surgery, these masses were found to be adherent to the dura. The lesions were excised, and histopathologic evaluation of each one revealed adamantinomatous craniopharyngioma (Fig 1C). Histologically, these were identical to the suprasellar craniopharyngioma resected in 1990 (Fig 1D). The patient received no further treatment and is doing well 1 year later.
Discussion
Craniopharyngiomas are neoplasms that arise from squamous epithelial rests along remnants of Rathke's cleft (1, 2). These most commonly arise in the suprasellar region (90%) and account for 1% to 3% of all intracranial neoplasms. More than half of all craniopharyngiomas occur in children and young adults. A second, smaller peak occurs in middle-aged adults. Patients may present clinically with headaches, visual disturbances, and hypothalamic and pituitary dysfunction (1, 3).
On gross pathology, most craniopharyngiomas are cystic masses with cholesterol-rich fluid and calcifications. Histologically, prominent epithelial lobules with palisading of cells at the periphery of the lobules and a stellate appearance of internal cells (so-called stellate reticulum) are seen (1, 2) (Fig 1C and D).
The hallmark of a craniopharyngioma on imaging studies is a suprasellar cyst with calcification and enhancement. CT scans typically reveal a cystic suprasellar mass with some solid component. Nodular or rim calcification is found in about 90% of cases. Attenuation of the cystic component is variable but is often slightly higher than CSF. Nodular or rim enhancement is seen on most contrast-enhanced CT studies. On MR imaging, a heterogeneous suprasellar mass with a highly variable appearance is observed, especially on T1-weighted images. A cyst with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images is the most common pattern (8, 9). High signal intensity on T1-weighted images may be seen, owing to high protein content or blood degradation products or both. Heterogeneous enhancement of the solid component has been noted on contrast-enhanced images (8–11).
Despite benign histopathologic features, craniopharyngiomas are locally invasive, with projections of tumor extending into the adjacent brain (2). Hence, total resection of this tumor is often difficult, and local recurrence at the primary site or in the contiguous brain may ensue, usually within 5 years (3, 4). A few cases of ectopic recurrence directly related to previous surgical procedures have been reported. These include frontal lobe implantation of craniopharyngioma along needle tracts following repeated suprasellar aspirations, sylvian fissure implantation directly adjacent to the surgical site, and craniopharyngioma recurring in the epidural space contiguous to the craniotomy site (5–7).
Conclusion
Our case represents a unique scenario of multiple ectopic dural-based metastatic foci of craniopharyngiomas remote from the primary site and contralateral to the craniotomy site, suggesting the possibility of meningeal seeding. Case reports of meningeal spread from other benign intracranial tumors, including meningioma, pituitary adenoma, choroid plexus papilloma, and central neurocytoma, have been reported (12–15); however, CSF seeding or distant metastases of craniopharyngiomas have not been reported to date. Long-term follow-up studies with MR imaging may cause this unique mode of tumor spread to be recognized in the future.
Footnotes
Presented at the annual meeting of the American Society of Neuroradiology, Philadelphia, May 1998.
Address reprint requests to Kanchan Gupta, MD, Department of Radiology, St. John's Hospital, 800 E Carpenter St, Springfield, IL 62769.
References
- 1.Petito CK, DeGirolami U, Earle KM. Craniopharyngiomas: a clinical and pathological review. Cancer 1976;37:1944-1952 [DOI] [PubMed] [Google Scholar]
- 2.Burger PC, Scheithauer BW. Tumors of the Central Nervous System: Atlas of Tumor Pathology.. Series 3, Fascicle 10. Washington, DC: Armed Forces Institute of Pathology; 1994:349–354
- 3.Sanford RA, Muhlbauer MS. Craniopharyngioma in children. Neurol Clin 1991;9:453-465 [PubMed] [Google Scholar]
- 4.Hetelekidis S, Barnes PD, Tao ML, et al. 20-year experience in childhood craniopharyngioma. Int J Radiat Oncol Biol Phys 1993;27:189-195 [DOI] [PubMed] [Google Scholar]
- 5.Barloon TJ, Yuh WTC, Sato Y, Sickels WJ. Frontal lobe implantation of craniopharyngioma by repeated needle aspirations. AJNR Am J Neuroradiol 1988;9:406-407 [PMC free article] [PubMed] [Google Scholar]
- 6.Ragoowansi AT, Piepgras DG. Postoperative ectopic craniopharyngioma: case report. J Neurosurg 1991;74:653-655 [DOI] [PubMed] [Google Scholar]
- 7.Malik JM, Rees Cosgrove GR, Vandenberg SR. Remote recurrence of craniopharyngioma in the epidural space: case report. J Neurosurg 1992;77:804-807 [DOI] [PubMed] [Google Scholar]
- 8.Pusey E, Kortman KE, Flannigan BD, Tsuruda J, Bradley WG. MR of craniopharyngiomas: tumor delineation and characterization. AJNR Am J Neuroradiol 1987;8:439-444 [DOI] [PubMed] [Google Scholar]
- 9.Ahmadi J, Destian S, Apuzzo MLJ, Segall HD, Chi-Shing Z. Cystic fluid in craniopharyngiomas: MR imaging and quantitative analysis. Radiology 1992;182:783-785 [DOI] [PubMed] [Google Scholar]
- 10.Eldevik OP, Blaivas M, Gabrielsen TO, Hald JK, Chandler WF. Craniopharyngioma: radiologic and histologic findings and recurrence. AJNR Am J Neuroradiol 1996;17:1427-1439 [PMC free article] [PubMed] [Google Scholar]
- 11.Donovan JL, Nesbit GM. Distinction of masses involving the sella and suprasellar space: specificity of imaging features. AJR Am J Roentgenol 1996;167:597-603 [DOI] [PubMed] [Google Scholar]
- 12.Zorludemir S, Scheithauer BW, Hirose T, Van Houten C, Miller G, Meyer FB. Clear cell meningioma: a clinicopathologic study of a potentially aggressive variant of meningioma. Am J Surg Pathol 1995;19:493-505 [PubMed] [Google Scholar]
- 13.Taylor WAS, Uttley D, Wilkins PR. Multiple dural metastases from a pituitary adenoma. J Neurosurg 1994;81:624-626 [DOI] [PubMed] [Google Scholar]
- 14.Leblanc R, Bekhor S, Melanson D, Carpenter S. Diffuse craniospinal seeding from a benign fourth ventricle choroid plexus papilloma. J Neurosurg 1998;88:757-760 [DOI] [PubMed] [Google Scholar]
- 15.Eng DY, DeMonte F, Ginsberg L, Fuller GN, Jaeckle K. Craniospinal dissemination of central neurocytoma. J Neurosurg 1997;86:547-552 [DOI] [PubMed] [Google Scholar]


