Abstract
BACKGROUND AND PURPOSE: Despite the continued improvements in endovascular techniques this decade, few dedicated studies addressing the feasibility of such procedures or their efficacy relative to risk have been conducted. The purpose of this study was to use current endovascular techniques to assess the feasibility, effectiveness, and safety of direct selective catheterization and embolization of the small branches of the cavernous segment of the internal carotid artery.
METHODS: We retrospectively reviewed the findings in 10 patients with lesions (five meningiomas and five arteriovenous malformations) primarily or partly supplied by branches of the meningohypophyseal trunk or inferolateral trunk who had undergone endovascular embolization of the feeding arteries during the period from 1991 to 1997. In each case, the artery was selectively catheterized with a microcatheter/microguidewire system and embolized with polyvinyl alcohol particles (n = 5), n-butyl cyanoacrylate tissue adhesive (n = 4), or both (n = 1).
RESULTS: In all 10 patients, the feeding artery from the meningohypophyseal trunk (eight patients) or inferolateral trunk (three patients; one patient with both) was successfully catheterized and embolized. In nine patients, embolization resulted in complete obliteration of the vascular territory; in the remaining patient, blood supply was decreased by an estimated 80%. No immediate or delayed complications occurred.
CONCLUSION: Advances in microcatheter and microguidewire technology allow more efficient and safer selective catheterization and embolization of branches of the cavernous segment of the internal carotid artery than in the recent past. Meticulous technique and detailed knowledge of the vascular anatomy of the cavernous sinus region are necessary to maximize lesion devascularization and to minimize the risk of stroke, cranial nerve palsies, and blindness.
Within the past decade, and particularly within the past 5 years, continued improvements in angiographic equipment, embolic agents, microcatheter and microguidewire technology, and operator experience have enabled smaller intracranial and extracranial vessels to be embolized in the treatment of a variety of vascular diseases, including pial-supplied meningiomas, hemangioblastomas, dural arteriovenous malformations (AVM), brain AVMs, and facial AVMs (1–5). Despite the resultant increase in the number and types of lesions treated by endovascular embolization of intracranial, spinal, or ophthalmic vessels of such small caliber, there have been few dedicated studies addressing the feasibility of such procedures or their efficacy relative to risk (2–4).
One difficulty in conducting and comparing interventional neuroradiologic studies is the rapid development of technology within the field; thus, experience, complication rates, and embolization efficacy may have changed dramatically from the late 1980s to the early 1990s, even for a particular group or person treating a specific type of lesion (6). The only dedicated series on cavernous internal carotid artery (ICA) vessel embolization is that by Halbach et al (4), who reported their 1984 to 1987 experience with seven patients (six with dural AVMs and one with a facial AVM).
These investigators (4) used a flow-directed catheter in their first case and a 3F-Tracker catheter (Target Therapeutics, Fremont, CA) and a 0.014-inch platinum steerable guidewire combination for the remainder. We believe that recent advances in microcatheter and microguidewire technology, including the variable stiffness hydrophilic microguidewires (such as the 0.016- and 0.010-inch Terumo goldtip hydrophilic wire; Target Therapeutics/Boston Scientific), the 0.014- and 0.011-inch hydrophilic shapeable Nitinol wires (such as the Transend EX 0.014-inch; Target Therapeutics/Boston Scientific), and the variable stiffness hydrophilic microcatheters (such as the Fast-Tracker-18 and Fast-Tracker-10), and the newer braided microcatheters (such as the TurboTracker, Excel-14, and Renegade; Target Therapeutics/Boston Scientific, and the Rapid Transit and Prowler-14; Cordis) allow more consistent and effective catheterization of these small cavernous segment branches. Between 1991 and 1997, we used these newer catheters and guidewires in conjunction with modern angiographic techniques, including high-resolution biplane digital roadmapping, to treat 10 patients (five with a meningioma, three with a brain AVM, and two with a dural AVM) whose lesions were also treated with direct embolization of branches of the meningohypophyseal trunk (MHT) and the inferolateral trunk (ILT) arising from the cavernous ICA. The results of this experience are reported here.
Methods
Ten patients (two men and eight women, ages 36 to 58 years) with lesions supplied in whole or in part by branches of the MHT (eight patients) or ILT (three patients; one with both ILT and MHT supply) underwent attempted selective catheterization and embolization. Treated lesions included five meningiomas and five AVMs (three pial, two dural). In each case, the lesions were fully characterized by cross-sectional imaging and diagnostic angiography before attempted embolization. Presenting symptoms were related to mass effect or hemorrhage and included headaches, visual symptoms, cranial nerve palsy, strokelike syndromes, and seizures. Two of the patients had already undergone attempted surgical resection of the lesion before embolization; none had undergone radiation therapy. A summary of the patients' demographic characteristics, disease, treatment techniques used, complications, and results of therapy appears in the Table.
Summary of 10 patients who underwent attempted selective catheterization and embolization between 1991 and 1997
In each case, selective angiography and embolization were performed with the patient under neuroleptic anesthesia. After catheterization of the cervical ICA with a 5F to 7F guiding catheter via a transfemoral route, the patient was systemically anticoagulated with 5000 to 7000 U intravenous heparin; adequate anticoagulation was confirmed by an activated clotting time of greater than 300 seconds. Heparinized saline was continually infused into the guiding catheter via the side arm of a rotating hemostatic valve. Selective catheterization was carried out using a variable stiffness 0.010- or 0.018-inch microcatheter (Tracker-18: 1.36-mm outer diameter [OD], 0.84-mm inner diameter [ID]; Tracker-18 extended tip: 1.15-mm OD, 0.84-mm ID; and Tracker-10: 1.0-mm OD, 0.56-mm ID) with a steerable hydrophilic microguidewire (Terumo 0.016-inch goldtip or Transend EX 0.014-inch). High-resolution digital fluoroscopy, digital subtraction angiography, and digital roadmapping were routinely used. Provocative testing with lidocaine or amobarbital sodium testing was not performed.
Depending on the anatomic and hemodynamic constraints and treatment goals of each case, embolization was carried out using either 50- or 150-μm polyvinyl alcohol (PVA) particles (one patient), 150- to 250-μm PVA particles (five patients), or tissue adhesive (five patients). Intraarterial nitroglycerin (50 μg/0.5 mL NS) was administered when it was thought that a component of vasospasm was limiting the effectiveness of the embolization. In cases of particulate use, when possible, embolization was carried out until complete stasis was achieved, with reflux of particles to the proximal ILT or MHT (reflux into the ICA was avoided).
In one patient (case 9), embolization was done with both tissue adhesive and particles. For particulate embolization, small volumes (0.1 to 0.3 mL) of a dilute suspension of PVA particles in nonionic contrast material were hand-injected using a 1-mL syringe during constant fluoroscopic monitoring for reflux. At the completion of embolization, as determined by near stasis of contrast agent, any particles remaining in the dead space of the catheter were flushed forward with normal saline while monitoring for reflux. Angiography was repeated with both selective hand and parent vessel injections to verify effective devascularization. For tissue adhesive embolization, n-butyl cyanoacrylate (NBCA) was mixed with iophendylate (Ethiodol) contrast (one-to-one in four cases, two-to-one in one case because of a high flow rate within the nidus of the AVM). Using multiple test injections under fluoroscopy after gauging the volume of contrast material required to opacify the target vessels, approximately three quarters of that volume of NBCA was loaded into the microcatheter and delivered to the target using the “sandwich” technique, in which delivery of the tissue adhesive bolus is preceded and followed by 5% dextrose solution in water (D5W) to prevent early polymerization. Between 0.1 and 1.0 mL of the NBCA:Ethiodol mixture was injected. The Tracker catheter was then immediately withdrawn from its seated position.
At the termination of the procedure, anticoagulation was reversed with intravenous protamine (50 to 100 mg). The patient was then placed on a standard postneuroembolization medical regimen that included corticosteroids (dexamethasone 4 mg IV every 6 hours), a calcium-channel blocker (nimodipine 60 mg orally every 4 hours), an antiemetic (Tigan 200 to 300 mg IM every 2 to 3 hours as needed, or Zofran 4 mg IV every 4 hours as needed), antacid (nizatidine or ranitidine 150 mg orally twice a day), and pain medicine for headache (morphine 3 to 7 mg IV every 3 to 4 hours as needed). Neurologic examinations with particular emphasis on cranial nerve function were performed immediately after recovery, the next morning, and during follow-up clinic visits.
Results
In all cases, selective catheterization of MHT or ILT branches was achieved. In nine of 10 patients, complete devascularization was demonstrated by postembolization angiography. In the 10th patient (case 3), who had a meningioma that was subsequently resected surgically, tumor supply was reduced by an estimated 80%. There were no immediate or delayed complications. In particular, no temporary or permanent cranial nerve deficits were found.
Three of the 10 patients received no further therapy after embolization (one patient with a dural AVM and two patients with pial AVMs, all treated with NBCA); five patients underwent postembolization surgical resection (four patients with meningiomas, all treated with PVA particles, and one patient with a dural AVM treated with NBCA); one patient underwent subtotal surgical resection followed by radiation therapy (a meningioma treated with PVA suspension), and one patient underwent postembolization radiation therapy alone (pial AVM treated with NBCA).
Representative Cases
Case 2
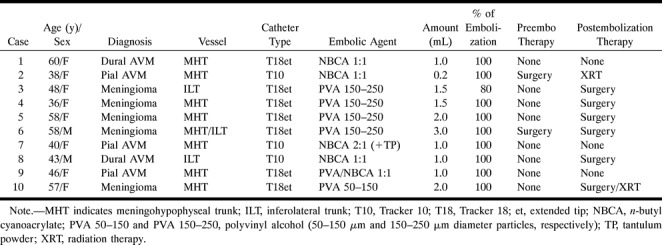
A 38-year-old woman who had undergone a craniotomy and direct glue embolization 13 years earlier for a ruptured pial AVM in the right occipital lobe presented with recurrent hemorrhage of the AVM. Diagnostic angiography and selective amytal testing were performed prior to a series of cerebral embolizations, including embolization of branches of the right anterior cerebral artery, right anterior choroidal artery, and right MHT (Fig 1). The MHT was selectively catheterized using a Fast-Tracker-10 catheter and a Dasher-10 wire with an S-shaped curve formed on the end of the microcatheter. Embolization was then performed using 0.2 mL of a mixture of 50% cyanoacrylate glue and 50% Ethiodol contrast, resulting in obliteration of the MHT supply to the AVM. The patient underwent postembolization linear accelerator radiosurgery with a dose of 1400 cGy. Follow-up MR imaging at 2 years showed no evidence of residual AVM. Symptoms related to the patient's recurrent hemorrhage resolved; there was no change in the bitemporal hemianopsia related to the remote hemorrhage and surgery.
fig 1.
Case 2: 38-year-old woman with recurrent AVM.
A, Lateral view during right internal carotid injection. An enlarged tentorial marginal branch of the MHT (closed arrow) and enlarged anterior choroidal artery (arrowheads) contribute to supply of the AVM (open arrow).
B, Selective right MHT injection after catheterization with a Tracker-10 catheter (arrow).
C, Repeat right ICA angiogram after embolization of the right MHT with 0.2 mL of cyanoacrylate glue/Ethiodol contrast mixture (1:1) shows complete obliteration of the MHT supply to the AVM (arrow).
Case 3
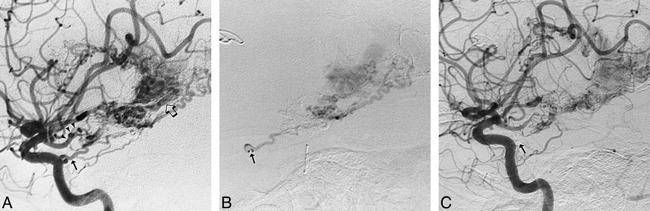
A 48-year-old right-handed woman presented with gradual loss of vision in the left eye. MR imaging revealed a 4-cm-diameter meningioma of the left sphenoid wing. Preoperative diagnostic angiographic embolization and balloon test occlusion were carried out (Fig 2). The left ILT was selectively catheterized using an extended tip Tracker-18 catheter with a 0.016-inch 75° Terumo goldtip wire, and embolization was performed with 150- to 250-μm PVA particles. A total of 1.5 mL was injected, resulting in approximately 80% reduction in the tumor blush. Subsequent subtotal surgical resection was uncomplicated, as was the patient's postoperative course.
fig 2.
Case 3: 48-year-old woman with a left sphenoid wing meningioma.
A, Left ICA injection in mid-arterial phase shows an enlarged ILT branch and the early blush from the sphenoid wing meningioma.
B, Late arterial phase shows a dense tumor stain, which persisted late into the venous phase.
C, Selective injection of left ILT after direct catheterization with an extended tip Tracker-18 catheter. Capillary phase confirms supply to the meningioma via the enlarged ILT (arrow).
D, Left ILT injection after embolization with 2 mL of 150 to 250 μm PVA suspension. Late arterial phase shows abrupt cutoff of the posterior branch of the enlarged MHT (closed arrow).
Case 5
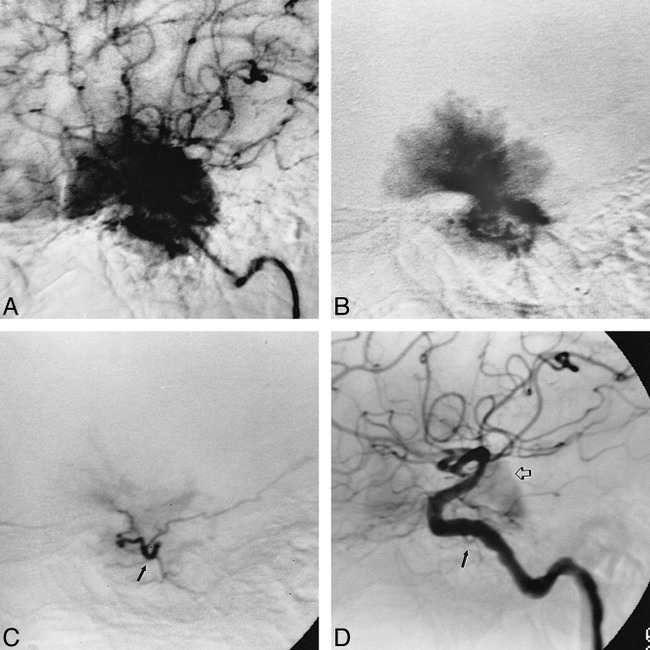
A 58-year-old right-handed woman presented with a 5-year history of left-sided dysfunction, including stumbling related to poor control of the left foot and poorly defined left body and face hypesthesia. MR imaging revealed a 1.5 × 2.5-cm left prepontine meningioma, exerting mass effect on the left pons and left cerebral peduncle. The patient underwent preoperative diagnostic angiography and embolization of the right MHT, as well as of the right ascending pharyngeal artery and right middle meningeal artery (Fig 3). The MHT was selectively catheterized using an extended-tip, Tracker-18 catheter with a 0.016-inch 70° Terumo goldtip wire. A total of 2 mL of contrast material with suspended 150- to 250-μm PVA particles was injected, resulting in near complete obliteration of supply to the tumor. Subsequent surgical resection of the lesion was uncomplicated. The patient's presenting symptoms resolved.
fig 3.
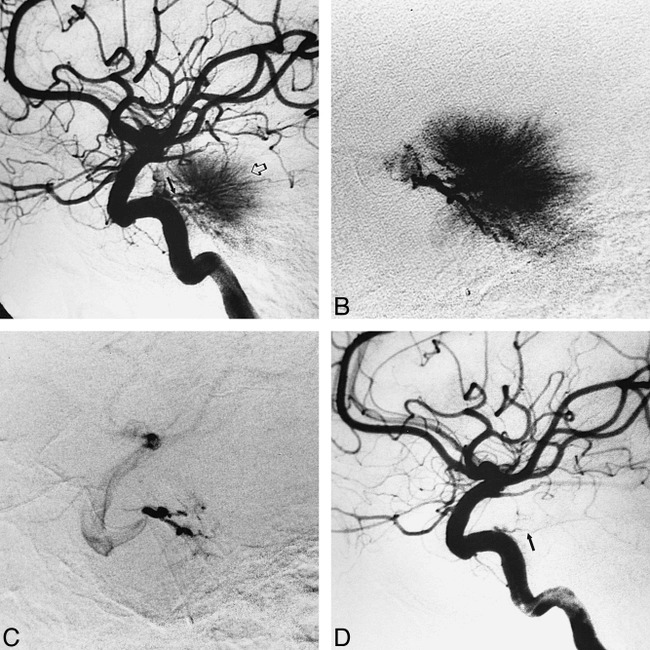
Case 5: 58-year-old woman with poor control of the left foot and left body hypesthesia.
A, Preembolization angiogram in late arterial phase during left ICA injection shows a dense tumor stain (open arrow) fed in part by left MHT branches (closed arrow).
B, Lateral view during selective injection in left MHT with an extended-tip, Tracker-18 catheter. Capillary phase confirms significant contribution to the tumor stain from left MHT.
C, Selective injection of left MHT after embolization with 2 mL of 150- to 250-μm PVA particle suspension shows near complete obliteration of the MHT supply to the tumor.
D, Left ICA injection after embolization confirms near complete devascularization of the tumor, with faint filling of the left tentorial marginal branch of the residual MHT (arrow).
Discussion
A thorough understanding of the anatomy of the branches of the cavernous segment of the ICA is a prerequisite for performing endovascular embolization in this critical vascular territory (7–16) (Fig 4).
fig 4.
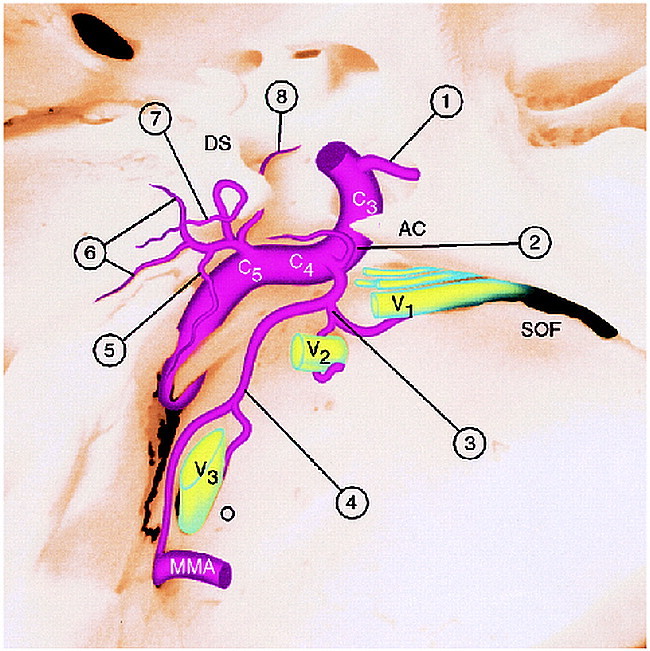
Diagram of the arteries arising from the cavernous segment of the ICA. AC, anterior clinoid; C3, C4, C5, segments of the intracavernous ICA; DS, dorsum sella; SOF, superior orbital fissure; O, foramen ovale; mma, middle meningeal artery; V1–V3, branches of the fifth cranial nerve coursing through superior orbital fissure (V1), foramen rotundum (V2), and foramen ovale (V3) accompanied by arterial branches of the ILT; 1, ophthalmic artery.
Branches of the ILT (C4 branches, 2 through 4):
2, Superior branch, supplying the roof of cavernous sinus; 3, anterior branch (to superior orbital fissure and foramen rotundum); 4, posterior branch (to foramen ovale and foramen spinosum).
Branches of the MHT (C5 branches, 5 through 8):
5, Recurrent artery of the foramen lacerum; 6, medial and lateral dorsal clival arteries; 7, tentorial marginal artery; 8, inferior hypophyseal artery.
In its complete form, the MHT projects posteriorly and laterally from its origin in the C5 segment (posterior genu) of the cavernous ICA, and quickly trifurcates into three branches: the tentorial marginal artery (of Bernasconi and Cassinari) (13), the dorsal meningeal artery (also known as the dorsal or lateral clival artery), and the inferior hypophyseal artery. Although the direct origin of some or all these arteries from the ICA may actually be more common than a true MHT configuration (15), these variations can be difficult to differentiate angiographically unless the individual branches are enlarged.
The dural branches arising from the C4 (horizontal) segment of the cavernous portion of the ICA are known collectively as the ILT. Anatomy of the ILT has also been well characterized (16) (Fig 4). It is a small vessel that can be difficult to recognize angiographically in healthy subjects, but can become enlarged with supplying AVMs, tumors, fistulas, or when acting as a collateral channel between the external carotid and internal carotid circulations. The ILT most commonly gives rise to three branches: a superior or tentorial branch supplying the roof of the cavernous sinus, the proximal portion of the third and fourth cranial nerves, and anterior and posterior branches, each which divide into medial and lateral rami, respectively. The anteromedial branch extends toward the superior orbital fissure, supplying distal portions of the third, fourth, and sixth cranial nerves and terminates as the deep recurrent ophthalmic artery, which anastomoses with the intraorbital ophthalmic artery. The anterolateral branch of the ILT enters the foramen of rotundum, anastomosing with the artery of the foramen of rotundum, a branch of the maxillary artery. The posteromedial branch of the ILT extends toward the foramen ovale, where it anastomoses with the accessory meningeal artery; it contributes to the supply of the sixth cranial nerve, the medial third of the gasserian ganglion, and the motor root of the fifth cranial nerve. The posterolateral branch of the ILT extends toward the foramen spinosum, where it anastomoses with the middle meningeal artery; it contributes to the supply of the middle and lateral third of the gasserian ganglion. As with the MHT, there is considerable variation in the origins of the arteries, which are commonly considered to be components of the ILT (16).
In view of the above anatomic considerations, it is evident that embolization of MHT or ILT branches carries a risk of ischemic injury to cranial nerves (10, 14), and this complication has been reported (17). Nevertheless, in our small number of patients, as well as in the series of seven patients reported by Halbach et al (4), embolization of these arteries was not accompanied by cranial nerve dysfunction, so that additional experience and larger series are required to establish the level of risk. Using particles larger than 150 μm has been suggested as a strategy to protect the small vasa nervorum, minimizing the risk of ischemic cranial nerve injury (18). We most commonly used PVA particles of 150 to 250 μm in this series, in which the most common objective was the preoperative embolization of meningiomas; however, with dural and pial AVMs, in which permanent devascularization is the objective, and if no dangerous collateral channels are identified, NBCA is the more desirable embolic agent, although use of NBCA carries at least a theoretical increased risk of permanent cranial nerve injury or monocular blindness. Provocative testing with intraarterial lidocaine injection has been advocated as a method to reveal whether occlusion of the tested artery will result in a cranial nerve deficit (19). In the setting of MHT or ILT embolization, however, there is a theoretical risk of intracranial reflux of lidocaine and resultant seizures, and false-negative as well as false-positive provocative tests have been reported in external carotid branch embolizations (4). Therefore, no provocative testing was performed in our patients.
It is our current practice to limit the use of liquid embolic agents (NBCA tissue adhesive) to those cases in which permanent devascularization of the lesion is the goal (ie, dural and pial AVMs) or to those in which no collaterals to the orbit are seen (such as the recurrent ophthalmic branch of the ILT) and a sufficiently distal catheter position can be achieved to avoid reflux into the ICA during test injections. Because the smallest drop of NBCA inadvertently embolized can result in a devastating complication if all the above requirements are not met, we limit our embolization to PVA particle suspension. Smaller particles (50 to 100 μm) can be used for maximum devascularization if a sufficiently selective catheter tip position is achieved. In the setting of preoperative embolization of meningiomas, we generally use slightly larger particles (150 to 250 μm), which decreases the risk of permanent ischemic injury of cranial nerves, prevents entry of particles into small collateral channels, and provides adequate devascularization for subsequent surgical resection.
In embolizing the branches of the ILT, the risk of embolization into the ophthalmic artery, via collateral flow through the deep recurrent ophthalmic artery, is a primary concern. This vessel in particular should be looked for on selective injections before embolization. If collateral flow to the ophthalmic artery is detected, embolization of the ILT may still be possible after occlusion of the collateral channel with an endovascular coil or gelfoam pledget (1), or flow-reversal techniques can be used, with slow embolization of particles into the target vessel with the catheter in a wedged or nearly wedged position, allowing reversed flow within the collateral channel to wash the particles into the target lesion. Obviously, in such a situation, the operator must carefully consider whether the potential benefit to the patient is worth the added risk of these maneuvers.
Although important anastomoses exist between the cavernous carotid branches and external carotid branches (15, 16), during direct MHT or ILT embolization these anastomoses are less of a concern than when embolizing external carotid branches, which can result in unintended embolization into ICA branches via these anastomoses. More important is the risk of stroke from reflux of embolic material intracranially during direct embolization of cavernous carotid branches.
Because the origins of the MHT and ILT are small and often arise at an acute angle, direct catheterization and seating can be difficult, posing considerable risk of reflux into the cerebral circulation. In addition, these sharp turns may be particularly vulnerable to perforation; one case report (20) documented the creation of an iatrogenic carotid-cavernous fistula after catheterization and embolization of the MHT. We believe these risks are minimized if the vessel is catheterized while working with the appropriate projection (perpendicular to the origin of the target vessel) and using high-resolution roadmapping and modern, softer microcatheters and microguidewires. It is our current practice to make our first attempt at catheterizing these small vessels with a 0.016-inch, 70° angled Terumo goldtip hydrophilic microguidewire and an unshaped TurboTracker-18 or TurboTracker-18 with a short dog-leg steamed at its tip. A fluoroscopic projection perpendicular to the origin of the target vessel is most useful, so that a lateral or slightly lateral oblique projection is used for MHT branches, and an anteroposterior or slight anteroposterior oblique projection is best for the ILT origin. If the above system is unsuccessful, our first alternative is a shapeable hydrophilic microguidewire (Transend 0.014- or 0.011-inch) with a tight C or S shape formed at the wire tip. At times, the stiffness of the radiopaque marker at the tip of the Tracker catheter (or the small but relatively stiff opaque tip of the Terumo goldtip) prevents selective catheterization of these small vessels with acute origins. In these cases the extended tip microcatheters, which have a relatively radiolucent but soft tip extending beyond the opaque marker, may allow entry into the vessel and provide sufficient purchase for careful, slow embolization with particles. If this fails, or when more distal positioning is required to allow safe embolization, it is usually necessary to resort to a 0.010-inch system (Tracker-10 and 0.010-inch Terumo goldtip or 0.011-inch Transend wire), although these smaller caliber catheters and wires at times do not appear to have the tensile strength to resist the force of flowing blood within the large lumen of the ICA, decreasing control and selectability. The more durable steam-shaped curves, which can be placed on the latest generation of braided microcatheters, such as the Excel-14, Rapid Transit, Prowler-14, and Renegade, have recently proved useful in delivering a microguidewire to the origin of these small target vessels. As a last resort, in cases in which the origin of the target vessel is directed inferiorly, at an acute angle, successful catheterization can sometimes be achieved by forming a Simmons-like curve on a shapeable microguidewire (0.014- or 0.011-inch Transend) and, after allowing this to form within the ICA and passing the wire beyond the target, directing the microcatheter into the origin of the target vessel by withdrawing the wire.
In their series of 10 ILT and MHT embolizations, Halbach et al (4) reported one case (the first of the series) that was complicated by embolic stroke. This was thought to be secondary to lack of systemic anticoagulation after direct cervical ICA access with a 5F catheter. A comparison of the technical aspects of our experience with that of Halbach et al almost a decade ago illustrates the rapid advances in microcatheter technology achieved over the past 5 to 10 years. Halbach et al used a flow-directed catheter in their first case and a 3F-Tracker catheter and 0.014-inch platinum steerable guidewire combination for the remainder. They meticulously removed the wire from the catheter frequently to prevent the formation of platelet thrombi on the guidewire tip and formed appropriate curves in the distal catheter tips to assist in catheterization. Eight of the 10 MHT or ILT branches were embolized successfully (degree of embolization not quantified) using similar agents to those used in our cases (one, NBCA; four, PVA 200- to 300-μm; two, D50). In our 10 patients, softer, smaller caliber, hydrophilic variable-stiffness microcatheters and hydrophilic steerable microguidewires were used with state-of-the art angiographic equipment, including high-resolution digital subtraction angiography with roadmapping. Although no procedure times were noted, it is our impression that these technologic improvements have significantly decreased the time needed to catheterize and embolize small branches such as the MHT and ILT selectively. With the newer hydrophilic guidewires, frequent removal of the guidewire is not necessary, and in fact can be detrimental, as the microcatheter can be damaged with multiple guidewire passes.
Conclusion
Using current endovascular techniques and equipment, we were able to embolize 11 of 11 cavernous branches successfully with no complications. Although more experience is required to establish the true risk of these procedures, our findings show that for meningiomas and AVMs supplied by branches of the MHT or ILT, current endovascular embolization methods and microcatheter technology enable appropriately trained interventionalists to embolize these small vessels effectively, efficiently, and safely.
Footnotes
Address reprint requests to Joseph M. Eskridge, MD, Department of Neurological Surgery, Box 356470, University of Washington, Seattle, WA 98195.
References
- 1.Nelson PK, Setton A, Choi IS, Ransohoff J, Berenstein A. Current status of interventional neuroradiology in the management of meningiomas. Neurosurg Clin N Am 1994;5:235-259 [PubMed] [Google Scholar]
- 2.Eskridge JM, McAuliffe WB, Harris DK, Kim DK, Scott J, Winn HR. Preoperative endovascular embolization of craniospinal hemangioblastomas. AJNR Am J Neuroradiol 1996;17:525-531 [PMC free article] [PubMed] [Google Scholar]
- 3.Terada T, Kinoshita Y, Yokote H, et al. Preoperative embolization of meningiomas fed by ophthalmic branch arteries. Surg Neurol 1996;45:161-166 [DOI] [PubMed] [Google Scholar]
- 4.Halbach VV, Higashida RT, Hieshima GB, Hardin CW. Embolization of branches arising from the cavernous portion of the internal carotid artery. AJNR Am J Neuroradiol 1989;10:143-150 [PMC free article] [PubMed] [Google Scholar]
- 5.Dowd CF, Halbach VV, Barnwell SL, Higashida RT, Hieshima GB. Particulate embolization of the anterior choroidal artery in the treatment of cerebral arteriovenous malformations. AJNR Am J Neuroradiol 1991;12:1055-1061 [PMC free article] [PubMed] [Google Scholar]
- 6.Wikholm G, Lundqvist C, Svendsen P. Transarterial embolization of cerebral arteriovenous malformations: improvement of results with experience. AJNR Am J Neuroradiol 1995;16:1811-1817 [PMC free article] [PubMed] [Google Scholar]
- 7.Dolenc VV, ed. Anatomy and Surgery of the Cavernous Sinus Wien: Springer; 1989
- 8.Parkinson D. Collateral circulation of the cavernous carotid artery: anatomy. Can J Surg 1964;7:251-268 [PubMed] [Google Scholar]
- 9.Schnurer LB, Statin S. Vascular supply of intracranial dura from internal carotid artery with special reference to its angiographic significance. Acta Radiol 1963;1:441-450 [Google Scholar]
- 10.Knosp E, Muller G, Perneczky A. The blood supply of the cranial nerves in the lateral wall of the cavernous sinus. In: Dolenc VV, ed. The Cavernous Sinus. New York: Springer; 1987
- 11.Inoue T, Rhoton Al, Theele D, Barry ME. Surgical approaches to the cavernous sinus: a microsurgical study. Neurosurgery 1990;26:903-932 [DOI] [PubMed] [Google Scholar]
- 12.Harris FS, Rhoton AL. Anatomy of the cavernous sinus. J Neurosurg 1976;45:169-180 [DOI] [PubMed] [Google Scholar]
- 13.Bernasconi V, Cassinari V. Un sengo carotidografico tipico di meningioma del tentorio. Chirurgia 1956;11:586-588 [Google Scholar]
- 14.Krisht A, Barnett DW, Barrow DL, Bonner G. The blood supply of the intracavernous cranial nerves: an anatomic study. Neurosurgery 1994;34:275-279 [DOI] [PubMed] [Google Scholar]
- 15.Lasjaunias P, Moret J, Doyon D, Vignaud J. C5 collaterals of the internal carotid siphon: embryology, angiographic anatomical correlations, pathological radio-anatomy.. Neuroradiology 1978;16:304-305 [DOI] [PubMed] [Google Scholar]
- 16.Lasjaunias P, Moret J, Mink J. The anatomy of the inferolateral trunk (ILT) of the internal carotid artery. Neuroradiology 1977;13:215-220 [DOI] [PubMed] [Google Scholar]
- 17.Capo H, Kupersmith MJ, Berenstein A, Choi IS, Diamond GA. The clinical importance of the inferolateral trunk of the internal carotid artery. Neurosurgery 1991;28:5:733-738 [DOI] [PubMed] [Google Scholar]
- 18.Latchaw RE. Preoperative intracranial meningioma embolization: technical considerations affecting the risk-to-benefit ratio (comment). AJNR Am J Neuroradiol 1993;14:583-586 [PMC free article] [PubMed] [Google Scholar]
- 19.Horton JA, Kerber CW. Lidocaine injection into external carotid branches: provocative test to preserve cranial nerve function in therapeutic embolization. AJNR Am J Neuroradiol 1986;7:105-108 [PMC free article] [PubMed] [Google Scholar]
- 20.Barr JD, Mathis JM, Horton JA. Iatrogenic carotid-cavernous fistula occurring after embolization of a cavernous sinus meningioma. AJNR Am J Neuroradiol 1995;16:483-485 [PMC free article] [PubMed] [Google Scholar]


