Fig. 4. Influence of mutations within TM domains 2 and 7 on the surface immunostaining of opsin and rhodopsin.
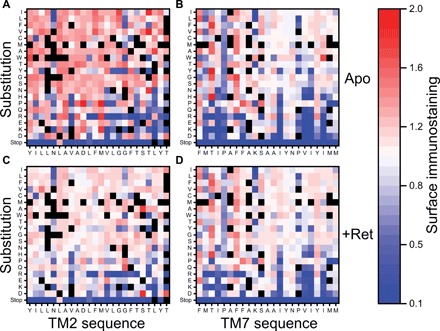
Surface immunostaning levels for rhodopsin variants bearing mutations within TMs 2 and 7 were determined by deep mutational scanning in the presence and absence of 9-cis-retinal and then normalized relative to the value of WT. Heatmaps depict the relative surface immunostaining values for opsin variants bearing each amino acid substitution (y-coordinate) at each position (x-coordinate) within TM2 (A) and TM7 (B) in the absence of retinal. Heatmaps depicting the relative surface immunostaining values for rhodopsin variants bearing each amino acid substitution within TM2 (C) and TM7 (D) in the presence of 5 μM 9-cis-retinal are also shown. Amino acids are arranged on the y-coordinate from the most hydrophobic (top) to the most polar (bottom) according to the White and von Heijne biological hydrophobicity scale (39). A value of 1.0 (white) corresponds to the surface immunostaining value for WT opsin/rhodopsin under each conditions. Variants that lack sufficient data for accurate quantification are indicated in black. Values reflect the averages from two biological replicates.
