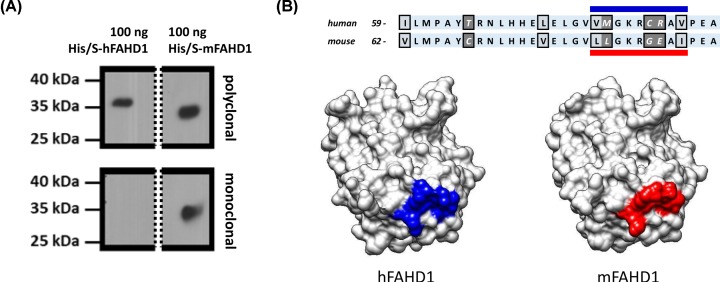
Figure 7. RabMab 27-1 against a defined epitope does not recognize hFAHD1.
(A) RabMab 27-1, raised against recombinant mFAHD1 was produced (see ‘Materials and methods’ section), that was found to recognize its antigen, but not hFAHD1. A Western blot (see ‘Materials and methods’ section) displays a clear differentiation with comparable concentrations of primary antibodies. Bands stem from the same gel and splice borders are indicated by the dashed line. Loaded FAHD1 was recombinant protein purified via His-tag and Ni-NTA affinity chromatography, following a defined protocol [7]. We find that both proteins display slightly different apparent protein masses on an SDS/PAGE gel. (B) Epitope analysis revealed that the region of interaction is situated in between amino acids 44 and 95 (Figure 6). Subsequent bioinformatics analysis, based on the available protein structures and sequences, suggest that the epitope is around the variant amino acid sequence L80 to I88 in mFAHD1 (V77 to I85 in hFAHD1), accounting for the major difference between the two structures.