Abstract
Cognitive decline can occur with normal ageing and in age‐related brain disorders, such as mild cognitive impairment and dementia, including Alzheimer's disease, with limited pharmacological therapies available. Other approaches to reduce cognitive decline are urgently needed, and so, the role of dietary interventions or nutraceuticals has received much attention in this respect. In this review, we examine the evidence for dietary plants and their chemical constituents as nutraceuticals, relevant to both cognitive decline in normal ageing and in dementia. Pharmacological (in vitro and in vivo), clinical and epidemiological evidence is assessed for both frequently consumed plants and their dietary forms, including tea, coffee, cocoa (chocolate), red wine, grapes, citrus and other fruits; in addition to plants used less frequently in certain diets and those that cross the blurred boundaries between foods, nutraceuticals and medicinal plants. For the latter, turmeric, saffron, sage, rosemary and lemon balm are examples of those discussed.
Linked Articles
This article is part of a themed section on The Pharmacology of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.6/issuetoc
Abbreviations
- AD
Alzheimer's disease
- Aβ
β‐amyloid
- BBB
blood–brain barrier
- BDNF
brain‐derived neurotrophic factor
- EGCG
epigallocatechin gallate
- H2O2
hydrogen peroxide
- MCI
mild cognitive impairment
- MMSE
Mini‐Mental State Examination
- PD
Parkinson's disease
- RCT
randomised controlled trial
- SAM
senescence‐accelerated mice
1. INTRODUCTION
The concept of food as medicine is as relevant today as it was in ancient Greece, where people believed that to restore “harmony” a correctly balanced diet would prevent or treat disease. Today, this principle is popularly applied to cognitive function with numerous claims for “brain boosting” foods. In this review, we examine the pharmacological evidence that chemicals in plant foods or nutraceuticals, can maintain, enhance normal or reverse declining, cognitive function. The evidence is based on peer‐reviewed reports of the effects of plant foods containing neuroactive chemicals in human (epidemiology and controlled clinical trial), animal (model) and laboratory (in vitro) studies.
Cognition is one of the most complex human capacities, consisting of “mental processes of receiving, choosing, transforming, storing, processing and retrieval of information, allowing the subject to navigate the world around him” (https://www.neuronup.com/en/areas/functions). While human studies depend on testing a range of relevant mental functions (using the Mini‐Mental State Examination [MMSE], for example), for animal models tests are generally confined to learning and memory, while for in vitro tests, surrogate markers such as indices of neurotransmitter, oxidative or inflammatory function are investigated.
Biomarkers depend on understanding mechanisms of normal and compromised cognition. As an example of how perilous this process can be, the deposition of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4865 (Aβ) has been considered for decades to be the central pathological mechanism and therapeutic target for Alzheimer's disease (AD), the main cause of cognitive decline in older people. But failed clinical trials of amyloidolytic agents indicate that the amyloid hypothesis may be incorrect (Herrup, 2015; Tse & Herrup, 2017). While for normal cognition, even the single process of information storage is not yet fully understood, though neurotransmitters (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=294 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1369), synaptic structures (dendritic spines) and neurophysiological responses (LTP) are among implicated mechanisms.
1.1. Cognitive decline in normal brain ageing
With ageing, some cognitive functions decline along with a degree of forgetfulness, decreased ability to maintain focus and solve problems, are common in the normal population. Biomarkers of the normal brain ageing process are not widely investigated, though oxidative stress (due to free radicals, for example), mitochondrial dysfunction, apoptosis and telomeric DNA depletion are implicated (van Ginneken, 2017), as they are in ageing in general, alongside increases in white matter lesions, Aβ plaques, neurofibrillary tangles, and tau pathology, Lewy bodies and α‐synucleinopathy, as well as decreasing neurotransmitter systems including cholinergic, vascular lesions and cortical (particularly hippocampal) shrinkage. These changes are increased in neurodegenerative diseases, each associated with specific cognitive disorders.
1.2. Cognitive decline in pathological brain ageing
The principle brain disorders that contribute to cognitive decline in ageing (with specific biomarkers) are early and late onset AD (Aβ42, total tau [T‐tau] and phosphorylated tau [P‐tau]), Lewy body dementia, Parkinson's disease (PD; synuclein and phosphorylated synuclein), vascular dementia, other vascular conditions and stroke (no such specific markers). In addition to disease‐specific protein abnormalities, other pharmacologically relevant biomarkers include indices of cholinergic, dopaminergic and serotonergic neurodegeneration (pre‐synaptic and post‐synaptic uptake or receptor sites and metabolic enzymes), abnormal neurotrophic function and neurogenesis, as well as a range of proteins involved in immune responses. A wide range of other protein abnormalities are currently investigated in neuroimaging, CSF and blood studies (Maclin, Wang, & Xiao, 2019; Olsson et al., 2016).
1.3. Mild cognitive impairment
In addition to major age‐related brain disorders, mild cognitive impairment (MCI) is diagnosed when memory impairment is beyond that expected for age and education but not in the dementia category. Biomarkers are complicated by the heterogeneity of MCI (Eliassen et al., 2017), which includes prodromal brain disease such as AD as well as benign, non‐progressive impairment. Added to this complex diagnosis is subjective cognitive decline with no objective neuropsychological dysfunction but considered to be a risk for abnormal cognitive decline and eventual progression to AD (Rabin et al., 2017).
With increasing awareness of age‐associated normal cognitive compromise and the risks of debilitating brain disease, prevention and treatment are now research priorities. Pharmacological interventions are currently limited (Brem & Sensi, 2018) and nutraceuticals are high on the list of options (Solfrizzi et al., 2018). The focus of this review is the pharmacology of plant foods or nutraceuticals, with known constituents that are relevant to cognition and brain ageing. Plant extracts of unknown composition or unknown concentration are included if they are supported by clinical and epidemiological studies. These may also harbour new clues for molecules and mechanisms involved in age‐related cognitive decline.
2. PHYTOCHEMICALS AS NUTRACEUTICALS
2.1. Turmeric
Populations that consume curry are reported to have a lower incidence of AD and healthy older people in these populations have better cognitive functions (Dominguez & Barbagallo, 2018). In support of these associations with diet, one study concluded that 60‐ to 90‐year‐olds (n = 1,010) that were frequent or occasional curry consumers had significantly higher MMSE scores, compared with non‐ or rare consumers (Perry & Howes, 2011). Consequently, there has been a plethora of studies to understand the mechanistic effects of turmeric (Curcuma longa) rhizome and its constituents, as a common curry ingredient. Most studies relevant to cognitive decline in dementia and normal ageing have focused on the curcuminoids, especially https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7000.
Oxidative stress has been linked with contributing to ageing and cognitive decline (Nelson & Tabet, 2015), and the antioxidant effects of curcumin have been of much interest in this respect. The curcumin enol structure with the intramolecular hydrogen bond is principally responsible for its free radical scavenging activity, with the phenolic hydrogens being essential for antioxidant activity and free radical kinetics. Curcumin can complex with metal ions via the diketone and pairs of phenol and methoxy groups of its structure, to reduce metal‐induced amyloid aggregation or oxidative neurotoxicity (Williams, Sorribas, & Howes, 2011). The antioxidant mechanisms of curcumin are also considered to explain its neuroprotective effects against Aβ, while related compounds from turmeric rhizome (demethoxycurcumin, bisdemethoxycurcumin and calebin A) are also neuroprotective in vitro (Howes & Simmonds, 2014; Williams et al., 2011).
Curcumin has been investigated in various animal models of cognitive decline including models of accelerated and normal ageing and dementia using different study designs, with benefits observed in most studies (Sanei & Saberi‐Demneh, 2019; Sarker & Franks, 2018; Williams et al., 2011). Its antioxidant mechanisms explain its ability to improve memory in many of these studies, including via reduced oxidative damage through enhancing https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6737 (GSH) and decreasing lipid peroxide levels in brain tissue (Perry & Howes, 2011; Sarker & Franks, 2018; Williams et al., 2011). Animal studies also show curcumin improves memory tasks and it can reverse stress‐induced reductions in neurogenesis, associated with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4872 (BDNF; Poulose, Miller, Scott, & Shukitt‐Hale, 2017). In the well‐established https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=330 model to assess memory retrieval in vivo, curcumin (50 or 100 mg·kg−1 orally for 10 days) restored the induced memory deficits in mice and prevented the scopolamine‐induced changes in Akt/glycogen synthase kinase‐3β, which is significant as the latter has been implicated in neurodegeneration and impaired cholinergic function (SoukhakLari, Moezi, Pirsalami, Ashjazadeh, & Moosavi, 2018). In the scopolamine animal model of memory impairment, demethoxycurcumin also ameliorated memory deficits, although in this study, this effect was correlated with a reversal of the reduced hippocampal https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2480 (ChAT) expression (Lim et al., 2016).
As the ageing process and neurodegenerative diseases have been characterised as pro‐inflammatory states (Nelson & Tabet, 2015; Poulose et al., 2017), anti‐inflammatory mechanisms of curcumin may also underpin its relevance to ameliorate cognitive decline (Table 1). Indeed, the molecular targets for curcumin are diverse and those relating to inflammatory mechanisms have been reviewed previously (Ghosh, Banerjee, & Sil, 2015; Nelson & Tabet, 2015). While the antioxidant and anti‐inflammatory mechanisms are the principal actions considered to explain curcumin's role against cognitive decline, it has shown other mechanisms more specifically associated with cognition in dementia. Curcumin produces different actions relevant to modulating Aβ (Table 1). Curcumin also maintains the function of protein disulfide isomerase, which catalyses maturation of disulfide bond‐containing proteins. Thus it prevents protein disulfide isomerase‐resistant misfolded protein forms that accumulate with oxidative stress that are implicated in the pathogenesis of AD (Howes & Perry, 2011). Relevant to both cognitive functions in ageing and dementia, curcumin rescued age‐related loss of hippocampal synapse input specificity of LTP via favouring https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75 activity (Phillips, 2017). With respect to the suggested link between hypercholesterolaemia and AD risk, the hypocholesterolaemic effects of curcumin, as observed in different animal studies, have also been suggested as an additional basis to reduce dementia risk and cognitive decline with ageing (Goozee et al., 2016).
Table 1.
Nutraceutical phytochemicals with relevance to cognition in normal and pathological brain ageing
| Phytochemical | Plant source(s) | Relevant bioactivities | Clinical effects/observations in humans (phytochemical or plant extract) |
|---|---|---|---|
| Epigallocatechin gallate (EGCG) and other catechins (flavan‐3‐ol monomers and oligomers [proanthocyanidins]) |
Tea, Camellia sinensis (L.) Kuntze Cocoa, Theobroma cacao L. Coffee, Coffea species (also in certain other edible plants, e.g. grapes, Vitis vinifera L.) |
EGCG ‐Antioxidant and neuroprotective against oxidative stress via elevation of mRNA expression of γ‐glutamylcysteine ligase to increase cellular GSH, stimulation of PKC and modulation of cell cycle genes (Williams et al., 2011) ‐Activates α7 nicotinic receptors and signalling molecules P13K and AKT to suppress Bcl‐2 down‐regulation (Howes & Simmonds, 2014) ‐Inhibits AChE, is anti‐inflammatory via different mechanisms and inhibits neuroinflammatory response of microglia to Aβ (Cascella et al., 2017) ‐Inhibits β‐ and γ‐secretase activities (Costa et al., 2017; Williams et al., 2011) Epicatechin ‐Increases GSH levels in astrocytes ‐Improves memory by ameliorating lipid peroxidation and ROS in rats with Aβ‐induced hippocampal toxicity (Williams et al., 2011) Cocoa flavanols ‐Enhance expression of neuroprotective and neuromodulatory proteins (Sokolov et al., 2013) ‐Improve angiogenesis and cerebral blood flow in the brain (Dominguez & Barbagallo, 2018) |
Green tea ‐Epidemiological evidence suggests regular intake reduced age‐related cognitive decline and dementia risk (Williams et al., 2011) ‐Intake (2 g daily for 3 months in an RCT) improved cognitive performance (Cascella et al., 2017) EGCG ‐Modulates cerebral blood flow and electroencephalographic activity in RCTs; effects not correlated with improved cognitive functions (Cascella et al., 2017) Chocolate (source of cocoa) ‐Intake for 2 years associated with lower risk of cognitive decline (41%; n = 531; persons aged ≥65 years; Dominguez & Barbagallo, 2018) ‐Habitual chocolate intake (age 23–98 years; n = 968) associated with improved cognitive performance (Crichton et al., 2016) ‐Cocoa flavanol intake for 3 months enhanced cognitive functions in healthy subjects (50‐ to 69‐year‐olds) ‐Cocoa flavanol intake increased brain perfusion and cerebral blood flow (in 50‐ to 65‐year‐olds; Dominguez & Barbagallo, 2018) Coffee (see below) |
| Caffeine |
Coffee Tea Cocoa |
Caffeine ‐Neuroprotective via inhibition of glutamate release and reduction of Aβ (Howes, 2013) ‐Stimulates PKC ‐Increases phosphor‐CREB levels and decreases phosphor‐JNK and ‐ERK expression mediating survival mechanisms in models of AD (Dominguez & Barbagallo, 2018) ‐Protects against BBB disruptions in animal models of AD and PD (Howes, 2013) |
Coffee ‐Dementia risk suggested as reduced in coffee drinkers compared with no or low coffee intake (Larsson & Orsini, 2018; Liu et al., 2016; Wu et al., 2017) ‐High serum caffeine levels in older persons with MCI associated with reduced progression to dementia (Dominguez & Barbagallo, 2018) ‐Mendelian randomisation study (>300,000 coffee drinkers) concluded no evidence that coffee intake could beneficially affect global cognition or memory (Zhou et al., 2018) |
| Caffeoylquinic acids (including chlorogenic acid) | Coffee |
Caffeoylquinic acids including chlorogenic acid ‐Suppress Aβ plaque deposition and prevent associated cognitive dysfunction in an AD model (Ishida et al., 2019) |
Caffeoyl‐ , dicaffeoyl‐ ,and feruloyl‐ quinic acids ‐Improved some cognitive functions in healthy participants (n = 38; 16 weeks) in an RCT (Saitou et al., 2018) ‐Improved memory functions in elderly with cognitive decline (n = 6; 6 months; Kato et al., 2018) Coffee (see above) |
| Resveratrol |
Grapes (also in blueberries, Vaccinium corymbosum L.; peanuts, Arachis hypogaea L.; and pistachios, Pistacia vera L.) |
Resveratrol ‐Anti‐Aβ: promotes decomposition of Aβ aggregates, prevents fibril formation, destabilises preformed fAβ42 in vitro, inhibits Aβ42 aggregation and reduces Aβ plaque formation (Williams et al., 2011) and aggregation and toxicity of amyloidogenic proteins (Costa et al., 2017) ‐Reduces cognitive deficits and is neuroprotective in vivo (Dominguez & Barbagallo, 2018; Howes & Perry, 2011) ‐Anti‐inflammatory via reduction of: astrocyte and microglial activation, NF‐κB activation, MAPK phosphorylation, TNF‐α and NO production, and COX‐2 expression (Mazzanti & Di Giacomo, 2016) ‐Improves and induces neurogenesis associated with improved levels of hippocampal BDNF mRNA (Shen et al., 2018) ‐Enhances proliferative states in neuronal stem cells in hippocampus (Phillips, 2017) |
Grape juice/ wine ‐Juice intake (12 weeks) improved verbal learning in older adults with cognitive decline (Phillips, 2017) ‐In 1,462 women (>34‐year study duration), wine consumption was associated with reduced dementia risk (Mehlig et al., 2008), yet separate study concluded a high red wine intake reduces AD risk in men but increases risk in women (Fischer et al., 2018) Resveratrol ‐Improved cognitive and cerebrovascular functions in postmenopausal women (n = 80; 75 mg twice daily; 14 weeks; Evans et al., 2017) ‐Improved cerebral blood flow in healthy and overweight individuals (n = 24–28; 250/500 mg daily for 21 days, to 75 mg daily for 12 weeks) but not cognitive functions (Mazzanti & Di Giacomo, 2016) ‐In AD patients (n = 119), intake (500 mg increased to 1 g twice daily for 52 weeks) reduced Aβ40 levels in plasma and CSF (Cicero et al., 2018); the dose taken by elderly people (n = 104) for 1 year showed less decline in some measures of cognitive functions in a double‐blind RCT (D'Cunha et al., 2019) ‐Low‐dose resveratrol with glucose and malate slowed scores of cognitive functions deterioration, although did not reach statistical significance (n = 39; Zhu et al., 2018) |
| Anthocyanins/ anthocyanidins |
Blueberries (also in certain other fruits including blackberries [Rubus species] and cherries [Prunus species]) |
Cyanidin ‐Reduces cell death, excitotoxicity, endoplasmic reticulum stress, and intracellular calcium release ‐Reduces ROS production induced by Aβ, glutamate, and H2O2 in cultured neurons (Thummayot et al., 2016) Anthocyanins ‐Attenuate oxidative stress in cerebral cortex and hippocampus (Pacheco et al., 2018) ‐Neuroprotective via phospholipase A2 inhibition (Mecocci et al., 2014) ‐Improve spatial memory in vivo and prevent scopolamine‐induced alterations in memory and restore brain ATP levels in vivo (Andres‐Lacueva et al., 2005; Gutierres et al., 2012); anthocyanidins and blueberry extracts improve cognitive functions in other animal models of memory impairment in aged animals (Mecocci et al., 2014) ‐Cyanidin 3‐O‐glucoside protects against Aβ‐induced impairment of learning and memory and attenuates tau hyperphosphorylation in vivo (Howes & Simmonds, 2014) |
Blueberries ‐RCTs (n = 26–37) showed oral intake (387 mg of anthocyanidins or 12 g of lyophilised blueberries) for 12 weeks to improve cognitive abilities in healthy older adults (Bowtell et al., 2017; Miller et al., 2018) ‐Extracts improve memory performance in older adults when taken for 3–6 months in RCTs (n = 94–122; McNamara et al., 2018; Whyte et al., 2018) ‐Prospective study associated blueberry (and strawberry, Fragaria species) intake with slower rates of cognitive decline in people aged >70 years (Mecocci et al., 2014) Cherries ‐Juice intake improved cognitive functions in dementia as observed in RCTs (Kelley et al., 2018) |
| Citrus flavanones, methoxyflavones, and coumarins | Citrus species including lemons, oranges, limes, and grapefruits |
Flavanones ‐Hesperidin and hesperetin attenuate cell death, excitotoxicity, oxidative stress, inflammation and mitochondrial dysfunction in cultured neurons (Hwang & Yen, 2008; Wang et al., 2013) ‐Naringenin improves spatial memory impairments, lipid peroxidation, and oxidative stress and decreases TNF‐α, TNF‐β, IL‐1, NF‐κB and caspase‐3 activation (Sachdeva & Chopra, 2015) Methoxyflavones ‐Nobiletin enhances memory and reduces tau hyperphosphorylation and oxidative stress in hippocampal tissue in vivo (Nakajima et al., 2013, 2015) ‐3,5,6,7,8,3′,4′‐Heptamethoxyflavone promotes BDNF production and activation of the cAMP/ERK/CREB cell survival signalling pathway (Sawamoto, Okuyama, Nakajima, & Furukawa, 2019) ‐Diosmin is neuroprotective and improves scopolamine‐induced memory impairments and reduces TNF‐α levels in vivo (Shabani & Mirshekar, 2018) Coumarins ‐Auraptene is neuroprotective and anti‐inflammatory via inhibition of microglial activation and COX‐2 expression (Okuyama et al., 2013) |
Citrus species ‐Flavonoid‐rich juices improve cognitive functions in healthy older adults (n = 37; 8‐week duration; Kean et al., 2015) and in healthy middle‐aged adults (n = 22) after acute administration (Alharbi et al., 2016) |
| Carotenoids include crocins (sugar esters of/or hydrolysed to, crocetin), β‐carotene, lutein and zeaxanthin |
Saffron, Crocus sativus L. (crocins/crocetin) Carotenoids widespread in higher plants include β‐carotene (e.g. occurs in carrots, Daucus carota L.) and also lutein and zeaxanthin, found in leafy vegetables |
Crocins ‐Inhibit Aβ formation, aggregation, and fibrillogenesis and are neuroprotective (Howes et al., 2017; Howes & Houghton, 2012) ‐Improve cognitive functions in different animal models of cognitive impairment in vivo ‐Protect against the neurotoxic effects of Aβ and glutamate in vitro (Bharate et al., 2018) ‐Anti‐inflammatory via inhibition of NO, TNF‐α and IL‐1β (Moshiri et al., 2015) Crocetin ‐Enhances antioxidant enzymes including GSH reductase and SOD and reduces lipid peroxidation in vivo (Hashemi & Hosseinzadeh, 2019) ‐Anti‐inflammatory via inhibition of NO, TNF‐α and IL‐1β (Moshiri et al., 2015) ‐Reverses Aβ‐induced memory deficits in vivo (Zhang et al., 2018) Saffron ‐Extracts inhibit AChE, although only by 30% (Bukhari et al., 2018) ‐Extracts improve cognitive functions in vivo (Bukhari et al., 2018; Christodoulou et al., 2015) |
Saffron ‐Extracts (30 mg daily; 16‐ or 22‐week duration) improve cognitive functions in AD patients in RCTs (Howes et al., 2017) ‐Cognitive functions improved in subjects with MCI after 1 year of saffron supplementation (n = 35; Tsolaki et al., 2016) Lutein and zeaxanthin ‐Dose of 10 and 2 mg, respectively, for 5 years by elderly people (n = 3,741) did not significantly improve cognitive functions in a double‐blind RCT (D'Cunha et al., 2019) β‐ Carotene ‐Daily intake (50 mg) for 1 year by elderly people (n = 4,051) improved measures of cognitive functions in a double‐blind RCT but only in the sub‐group that had previously participated in a separate study involving aspirin intake (D'Cunha et al., 2019) ‐Supplementation for 18 years associated with improved cognitive performance in men compared with placebo (Mecocci et al., 2014) |
| Curcuminoids including curcumin | Turmeric, Curcuma longa L. |
Curcumin ‐Anti‐inflammatory: reduces NF‐κB, TNF‐α, and COX‐2 signalling pathways and inhibits IL‐8, Il‐6, IL‐1β and IL‐1α production via inhibition of the MAPK and NF‐κB pathways (Ghosh et al., 2015; Nelson & Tabet, 2015) ‐Antioxidant: reduces oxidative damage by enhancing GSH and decreasing lipid peroxide levels in brain tissue (Williams et al., 2011) ‐Neuroprotective: inhibits Aβ formation and shows protective effects in models of AD (Poulose et al., 2017) ‐Improves cognitive functions in different animal models of memory impairment (SoukhakLari et al., 2018; Williams et al., 2011) ‐Anti‐Aβ: inhibits formation of Aβ oligomers, inhibits β‐secretase, binds to existing plaques, reduces Aβ in vivo, and inhibits Aβ‐induced tau phosphorylation to reduce microglial activation (Goozee et al., 2016; Williams et al., 2011) ‐Reverses age‐related loss of hippocampal synapse input specificity of LTP via favouring NMDA receptor activity (Phillips, 2017) |
Curcumin ‐Improvements in scores for cognitive functions not observed after 6 months of 1 or 4 g of curcumin daily in AD patients (n = 34) or after 6 months of 2 or 4 g of curcuminoids (95%) daily in an RCT followed by 6 months as an open‐label trial in dementia patients (n = 36; Dominguez & Barbagallo, 2018) ‐Healthy older adults (age 60–85 years; n = 60) had improved attention and working memory following acute administration and improved working memory and mood following 1 month of administration, of 400 mg·day−1 (Cox et al., 2015) ‐Older adults (n = 96) taking 1,500 mg·day−1 in a double‐blind RCT for 12 months did not have improved cognitive functions (Rainey‐Smith et al., 2016) |
| Monoterpenes |
Occur in various aromatic herbs and spices including those in the Lamiaceae family, e.g. common sage, Salvia officinalis L.; Spanish sage, S. officinalis subsp. lavandulifolia (Vahl) Gams; rosemary, Salvia rosmarinus Spenn.; lemon balm, Melissa officinalis L.; and mints, Mentha species Also in spices such as black cumin, Nigella sativa L. |
Monoterpenes ‐AChE inhibition: 1,8‐cineole and α‐pinene are uncompetitive reversible inhibitors (IC50 0.67 and 0.63 mM, respectively (Howes & Perry, 2011) ‐Nicotinic receptor activity: 1,8‐cineole, α‐pinene, camphor, and borneol (Wake et al., 2000) ‐Thujone inhibits AChE yet impairs memory in a laboratory model by inhibiting ACh via nicotinic receptors (Wake et al., 2000) ‐Thymoquinone (occurs in black cumin) is a nicotinic α7 receptor agonist (Ibrahim et al., 2016) 1,8‐ Cineole ‐Anti‐inflammatory: suppresses LPS‐induced pro‐inflammatory cytokine production (via NF‐κB, TNF‐α, IL‐1β and IL‐6 and ERK pathways; Howes, 2018; Seo, Fischer, & Efferth, 2018) ‐Antioxidant: reduces oxidative stress in neurodegenerative disease models via ROS scavenging (Porres‐Martínez et al., 2016; Kim, Seo, Min, Park, & Seol, 2014) ‐Modulates TRP (transient receptor potential) channels (including increasing spontaneous glutamate release); dysregulation of which have been suggested to be involved in neurological and psychiatric disorders (Jiang et al., 2016) |
1,8‐ Cineole ‐Crosses the BBB, including via inhalation; 1,8‐cineole blood levels have been correlated with increased cognitive performance (Okello & Howes, 2018) Lamiaceae plants ‐Both common and Spanish sage extracts enhance cognition (memory and alertness) in healthy, young, and older people and counter cognitive impairment and improve behavioural measures in people with AD (Perry & Howes, 2011) ‐Common sage, lemon balm, and rosemary combined alcohol extracts improved word recall in <63‐year‐olds in a controlled trial (Perry et al., 2018) ‐Rosemary extracts improve attention and memory in healthy adults and in the elderly (Nematolahi et al., 2018) ‐Rosemary oil and lavender (Lavandula angustifolia Mill) oil improve cognitive functions in people (Okello & Howes, 2018) ‐Peppermint (Mentha × piperita L.) oil (oral) improved performance in demanding cognitive tasks and attenuated the increase in mental fatigue associated with extended cognitive task performance in healthy adults (Kennedy et al., 2018) ‐Inhalation of peppermint volatile components enhances memory and alertness (Okello & Howes, 2018) ‐Spearmint extract (Mentha spicata L.) improved measures of reactive agility in a double‐blind RCT in healthy adults (Falcone et al., 2018) ‐Lemon balm extracts improve cognitive processing, memory, and attention in healthy adults and mood in AD in controlled trials (Howes & Houghton, 2012) Black cumin ‐Enhances cognition, verbal learning, memory, and attention in the elderly (Bin Sayeed et al., 2014) |
| Rosmarinic acid | Occurs in the Lamiaceae family, including in common sage, Spanish sage, rosemary, lemon balm and mints |
Rosmarinic acid ‐Inhibits AChE ‐Anti‐Aβ via different mechanisms ‐Antioxidant ‐Neuroprotective (Williams et al., 2011; Perry & Howes, 2011; Howes & Houghton, 2012; Mecocci et al., 2014) |
Lamiaceae plants (see above for studies on extracts) |
| Isoflavones include https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2826 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2828 | Soya beans, Glycine max (L.) Merr. |
Isoflavones ‐Mimic effects of estrogen in the brain; estrogenic effects suggested to improve cholinergic function ‐Influence synthesis of ACh and neurotrophic factors ‐Reduce age‐related neuronal loss and cognitive decline in vivo ‐Improve cognitive functions in other animal models of memory impairment ‐Antioxidant via increased GSH peroxidase and decreased lipid peroxidation ‐Genistein is neuroprotective against Aβ‐induced neurotoxicity and ameliorates Aβ‐induced hippocampal neuronal apoptosis in vitro (Mecocci et al., 2014; De Domenico and Giudetti, 2017; Howes & Houghton, 2009) |
Isoflavones ‐A high‐soya diet (100 mg of total isoflavone daily for 10 weeks) in student volunteers was suggested to improve short‐ and long‐term memory in both females and males ‐Consumption of soya isoflavones by postmenopausal women for a period of 12 weeks improved cognitive functions, and in a double‐blind RCT, some cognitive benefits, particularly verbal memory, occurred in postmenopausal women taking isoflavone supplements for 6 months (Howes & Houghton, 2009) ‐Long‐term (30 months) supplementation in women showed no benefits on global cognition but improved visual memory ‐12‐week supplementation in men improved spatial working memory only in men (Mecocci et al., 2014) |
Curcumin has been evaluated clinically but has not consistently resulted in cognitive improvements. In AD patients (n = 34), 1 or 4 g of curcumin daily had no significant effects on MMSE after 6 months compared with placebo. Dementia patients (n = 36) taking 2 or 4 g of curcuminoids (95%) daily for the same period in a randomised controlled trial (RCT), followed by another 6 months as an open‐label trial, also showed no significant improvements in scores of cognitive functions (Dominguez & Barbagallo, 2018). The lack of clinical efficacy may be attributed to the poor bioavailability of curcumin. Furthermore, clinical studies have been focused on the effects of curcumin in dementia patients, with neurodegeneration and other pathological features at an advanced stage; in such circumstances, improving cognitive functions may be more difficult to achieve, especially with a substance of low oral bioavailability.
In a recent meta‐analysis of trials with curcumin intervention in different groups of individuals, it was concluded that curcumin appears to be more effective in improving cognitive functions in the elderly, rather than in those with AD (Zhu, Mei, Zhang, Xie, & Lang, 2019). In one of the limited number of studies in this respect, a double‐blind RCT with healthy older participants age 60–85 years (n = 60) revealed that 400 mg·day−1 of curcumin (an optimised formulation, Longvida) could improve attention and working memory following acute administration and both working memory and mood following 1 month of administration (Cox, Pipingas, & Scholey, 2015). However, in another double‐blind RCT, ingestion of 1,500 mg·day−1 of curcumin (Biocurcumax™) by older adults (n = 96) for 1 year did not conclusively improve cognitive functions (Rainey‐Smith et al., 2016).
The pharmacological activities of curcumin have not to date been effectively translated to clinical efficacy to prevent or ameliorate cognitive decline with normal ageing or in dementia. Yet the mounting preclinical evidence for the mechanistic effects of curcumin provide hope that with rigorous studies to evaluate its effects on cognitive functions in the general ageing population and in relation to AD risk, with formulations providing higher bioavailability, curcumin may still have a role in maintaining cognitive ability with ageing. Indeed, the spice, pepper (Piper nigrum) is a source of the alkaloid https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2489, which increases bioavailability of curcumin by 2,000% (Shoba et al., 1998), indicating that certain dietary plants in combination should be scientifically evaluated more extensively.
2.2. Grapes and red wine
Early epidemiological studies associated wine intake with a reduced dementia risk, although it has been suggested that this evidence should be combined with analysis of other risk factors such smoking and ApoE4 status (Perry & Howes, 2011). In a 34‐year follow‐up study of 1,462 women, wine consumption was associated with a reduced dementia risk, but consumption of spirits was suggested to increase dementia risk (Mehlig et al., 2008). However, more recent studies conclude that a higher red wine intake only reduces AD risk in men but increases risk in women (Fischer et al., 2018). Although the effects of wine and grapes (Vitis vinifera) on dementia risk are mixed, potential benefits have largely been attributed to the component, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8741 (trans‐3,4′,5‐trihydroxystilbene). Although most dietary resveratrol is from grapes and wine (Dominguez & Barbagallo, 2018), it also occurs in a range of other dietary sources, including peanuts, pistachios and blueberries (Howes & Perry, 2011). Consequently, there has been extensive research to understand the mechanistic effects of resveratrol relevant to both cognitive functions with ageing and in dementia.
With respect to dementia pathology, resveratrol is anti‐Aβ via different mechanisms (Table 1). Resveratrol is a more potent inhibitor of Aβ42 aggregation compared with certain other plant‐derived antioxidants (resveratrol > catechin > curcumin > piceid > ginkgolides) and it reduces Aβ plaque formation in a transgenic model of AD (Williams et al., 2011). It also reduces expression of transthyretin, a carrier protein that reduces the aggregation and toxicity of amyloidogenic proteins (Costa et al., 2017). Furthermore in a mouse model of AD, resveratrol increased life expectancy and reduced cognitive deficits, in addition to being neuroprotective and reducing both Aβ burden and tau hyperphosphorylation (Howes & Perry, 2011).
Anti‐inflammatory mechanisms of resveratrol are summarised in Table 1 and may contribute to the benefits of this stilbene against cognitive decline in dementia. Indeed, the altered amyloid precursor protein processing and anti‐inflammatory and antioxidant effects of grape supplementation (containing resveratrol, catechins, flavonols and phenolic acids: ferulic, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5549 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5155 acids) in an animal model of AD were concluded to explain its ability to improve cognitive deficits (Sheng et al., 2018). Gallic acid alone also inhibits formation of Aβ42 aggregates to improve cognitive functions in an AD animal model (Yu et al., 2019), further demonstrating that resveratrol is not the only active constituent of grape products in the diet or as nutraceuticals. Resveratrol also protects against oxidative stress, reduces hippocampal neurodegeneration and increases memory performance in other animal models of cognitive impairment (Dominguez & Barbagallo, 2018; Howes & Perry, 2011).
As with many other dietary polyphenols, resveratrol has antioxidant effects that are suggested to underpin its neuroprotective action, relevant to cognition. In rat hippocampal slices, resveratrol protects astrocytes from hydrogen peroxide (H2O2)‐induced oxidative stress by increasing glutathione (GSH) levels (Williams et al., 2011). Resveratrol also scavenges reactive oxygen species (ROS) but not as potently as its metabolite piceatannol (3′,4′,3,5‐tetrahydroxystilbene), which occurs in similar dietary sources but at lower concentrations (Williams et al., 2011). Both stilbenes could therefore contribute to the effects on cognitive functions of grape products, although piceatannol has not been studied as extensively as resveratrol.
It is too simplistic to conceive that antioxidant mechanisms alone are responsible for the effects of resveratrol and other mechanisms may also contribute to improved cognitive functions and neuroprotection. Its other mechanisms relevant to cognition include activation of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2707, a histone deacetylase that is involved in responding to molecular damage and metabolic imbalances (Howes & Perry, 2011). In mice, resveratrol (40 mg·kg−1 daily for 1 month) induced neurogenesis, which was associated with improved levels of hippocampal BDNF mRNA, compared with controls. Grapeseed extract also improves adult neurogenesis in vivo (Poulose et al., 2017) and so may also be of interest as a potential nutraceutical for maintaining cognitive functions. Resveratrol's neuroprotective effects may also be due to activation of the sirtuin 1 miR‐134 pathway and up‐regulation of CREB/BDNF expression (Shen, Xu, Qu, Sun, & Zhang, 2018). Another study revealed that resveratrol enhanced proliferative states in neuronal stem cells in rat hippocampus and via BDNF restoration, it attenuated stress‐induced learning deficits and hippocampal degeneration in vivo (Phillips, 2017).
Resveratrol as a nutraceutical has been investigated clinically. One RCT (n = 80) showed that 14 weeks of resveratrol supplementation (75 mg twice daily) could improve cognitive and cerebrovascular functions in postmenopausal women (Evans, Howe, & Wong, 2017). Some small studies (n = 24–28) demonstrated that resveratrol can improve cerebral blood flow in healthy and overweight individuals at doses ranging from 250 or 500 mg daily for 21 days to 75 mg daily for 12 weeks, although cognitive functions were not improved (Mazzanti & Di Giacomo, 2016). When taken for a longer period of 26 weeks, however, 200 mg of resveratrol daily combined with the flavonol quercetin improved memory retention in healthy overweight individuals (n = 46). This flavonol and its derivatives are common to many dietary plants including grapes and, interestingly, has also shown numerous mechanistic effects relevant to AD and cognition, including antioxidant, neuroprotective and cytokine‐regulating actions (Zaplatic, Bule, Shah, Uddin, & Niaz, 2019); its 3‐O‐glucuronide metabolite has been shown to reduce Aβ peptide generation and AD‐type deficits (Howes & Simmonds, 2014) and thus may also contribute to the effects of grapes and wine on cognitive ability with ageing and dementia. Furthermore, in line with this finding, grape juice intake for 12 weeks improved verbal learning in older adults with cognitive decline, although this was not observed in those with dementia (Phillips, 2017). Another study suggested that in subjects with mild cognitive decline (n = 10), a grape formulation could protect against metabolic decline in brain regions that are affected in early AD, compared with a placebo (Lee, Torosyan, & Silverman, 2017).
While studies on cognitive functions in healthy subjects show some promise, efficacy in dementia patients has not been comprehensively evaluated. One cohort study to investigate factors associated with ageing concluded that levels of urinary resveratrol metabolites could not be correlated with inflammation, cardiovascular disease (a risk factor for AD and cognitive decline) or all‐cause mortality; this outcome was considered to be due to the typically low levels of resveratrol in the diet (Nelson & Tabet, 2015). In AD patients (n = 119), resveratrol intake (500 mg daily with increased doses every 13 weeks for 52 weeks, to 1,000 mg twice daily) reduced Aβ40 levels in plasma and CSF, while it was also concluded to be safe and well tolerated at the doses tested (Cicero, Fogacci, & Banach, 2018). A very low dose of resveratrol compared with doses used in other studies has been evaluated in combination with glucose and malate to determine any effect on slowing AD progression. The rationale for this combination is that glucose and malate prime oxidative metabolism and the Krebs cycle in the brain, to aid in regenerating the reduced form of resveratrol under normal brain cell metabolism. After 12 months of this treatment, scores of cognitive functions showed less deterioration in the treatment group compared with controls (n = 39), although observed differences did not reach statistical significance (Zhu et al., 2018). However, both the treatment group and the control group in this study took the treatment or placebo in 8 oz of unsweetened grape juice and since resveratrol and other polyphenols occur in grapes/grape juice with mechanistic effects relevant to cognitive functions, the influence of this preparation on the study outcome should also be considered. Further trials are in progress to evaluate the effects of resveratrol alone on cognitive functions (Mazzanti & Di Giacomo, 2016). These may provide new insights into the role of resveratrol in the diet or as a supplement in preventing cognitive decline with ageing or in age‐related diseases.
2.3. Berry and citrus fruit
Numerous plant families produce fruits identified as berries, including the Ericaceae and Rosaceae; the former includes species of the genus Vaccinium commonly called cranberries, bilberries, lingonberries or blueberries, while the latter family involves Fragaria species known as strawberries, Crataegus or hawberries and Rubus species, known as raspberries or blackberries. Citrus fruits (genus Citrus in the Rutaceae) include cultivated fruits such as lemons, oranges, limes and grapefruits. Berry and citrus fruits contain various phytochemicals including flavonoids, anthocyanidins/anthocyanins, tannins, phenolic acids, coumarins and terpenoids, compounds extensively documented for their potential effects on health and cognition (Howes & Simmonds, 2014; Jimenez‐Garcia et al., 2013; Smeriglio, Barreca, Bellocco, & Trombetta, 2017). For example, protocatechuic acid reduces cell death, excitotoxicity, endoplasmic reticulum stress, intracellular calcium release and ROS production induced by Aβ, glutamate and H2O2 in cultured neurons; the anthocyanidin, cyanidin, produces similar effects (Table 1; Hornedo‐Ortega et al., 2016; Thummayot, Tocharus, Suksamrarn, & Tocharus, 2016; Winter et al., 2017). Different methoxyflavones and flavanones from citrus fruit also show pharmacological actions relevant to cognition and dementia (Table 1). Thus, different berry and citrus fruit constituents mediate a range of mechanistic effects that are relevant to cognitive functions, indicating that such mixtures may be more useful as nutraceuticals, rather than single isolated chemicals in some circumstances.
Citrus species are a source of lipophilic monoterpene chemicals and some of these may influence mood and cognitive functions (Okello & Howes, 2018); they include monoterpenes that occur in other plant families (e.g. Lamiaceae) that have shown mechanistic effects relevant to cognition (see below and Table 1). However, the hydrophilic character of certain berry and citrus flavonoids and the degree of glycosylation (sugars attached to aglycone phytochemicals) have raised questions about the relevance of in vitro studies, in relation to the bioavailability and metabolism of such compounds, in vivo. Conjugated metabolites may also be transformed by resident bacteria through hydrolysis of glycosides, glucuronides, sulfates, amides, esters and lactones or by modifications of flavonoid rings (Selma, Espin, & Tomas‐Barberan, 2009); thus, the ingested phytochemicals may not represent the metabolites that can reach the CNS to mediate effects on cognitive functions. For example, the levels of ingested anthocyanins detectable in plasma have been reported as less than 2%, although it has been suggested that the bioavailability of this compound class may have been underestimated (Lila, Burton‐Freeman, Grace, & Kalt, 2016). With respect to blood–brain barrier (BBB) permeability, in vitro BBB models show that some flavonoids and their metabolites can be transported across hCMEC/D3 and RBE‐4 cells (Faria et al., 2010).
With consideration of the metabolism and bioavailability of citrus and berry flavonoids, several studies using animal models of cognitive decline have demonstrated the neuroprotective effect of fruit extracts and compounds. Several studies have focused on blueberries (Vaccinium species) in this respect. One study revealed that a 2% blueberry diet in F344 aged rats for 8 to 10 weeks improved spatial memory, an outcome that was correlated with the concentration of anthocyanins detected in cerebellum, striatum, cortex and hippocampus (Andres‐Lacueva et al., 2005), indicating adequate oral and BBB bioavailability to mediate cognitive effects. A separate study concluded that a blueberry (Vaccinium ashei; synonym of Vaccinium corymbosum) extract administered orally for 30 days to mice enhanced long‐term memory and protected against brain DNA damage induced by ROS (Barros et al., 2006). Anthocyanins given orally to rats for 25 days attenuated oxidative stress in cerebral cortex and hippocampus induced by intracerebroventricular streptozotocin (Pacheco et al., 2018), while their intraperitoneal administration to rats for 1 week prevented scopolamine‐induced alterations in memory and restored brain ATP levels (Gutierres et al., 2012). Other anthocyanin‐containing fruit also under evaluation for effects against cognitive decline include cherries (Prunus species; Rosaceae); extracts or polyphenol/anthocyanin constituents improved memory in vivo and in short‐term RCTs, intake of cherry juice improved cognitive functions in patients with dementia (Kelley, Adkins, & Laugero, 2018).
Citrus flavonoids have been investigated for their effects on cognitive functions in different animal models. The citrus flavanone https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10298 given orally to rats for 14 days improved spatial memory impairments, lipid peroxidation and oxidative stress induced by intracerebroventricular okadaic acid, and it decreased https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074, TNF‐β, IL‐1, NF‐κB, and caspase‐3 activation (Sachdeva & Chopra, 2015). The hexamethoxyflavone nobiletin enhanced memory and reduced tau hyperphosphorylation and oxidative stress in the hippocampal tissue of senescence‐accelerated mice (SAM) after intraperitoneal administration for 40 days (Nakajima et al., 2013) and when administered by the same route for 3 months to mice (AD model), memory was improved and oxidative stress was reduced, although no effect on brain Aβ levels was observed (Nakajima et al., 2015). These studies indicate that nobiletin has adequate BBB permeability to mediate effects on cognition, perhaps due to the high degree of methoxylation on the flavone structure. Another citrus flavonoid, diosmin (the 7‐rutinoside of the flavone diosmetin), was neuroprotective in vivo after 7 days of administration (intraperitoneal) to rats and it improved scopolamine‐induced memory impairments and reduced TNF‐α levels (Shabani & Mirshekar, 2018). A different class of compound from citrus, the coumarin auraptene, is also neuroprotective in vivo and mediates effects on inflammatory pathways including inhibition of microglial activation and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1376 expression by astrocytes in the CNS (Okuyama et al., 2013).
The mechanistic effects of blueberry and citrus fruit constituents may explain effects on cognitive functions that extracts from these plants have with respect to their intake as nutraceuticals. Small RCTs (n = 26–37) with blueberry nutraceuticals have shown oral intake of a concentrate containing either 387 mg of anthocyanidins or 12 g of lyophilised blueberries for 12 weeks improves cognitive abilities in healthy older adults (Bowtell, Aboo‐Bakkar, Conway, Adlam, & Fulford, 2017; Miller, Hamilton, Joseph, & Shukitt‐Hale, 2018). Larger RCTs (n = 94–122) also conclude that blueberry extracts improve memory performance in older adults when taken for periods of 3 to 6 months (McNamara et al., 2018; Whyte, Cheng, Fromentin, & Williams, 2018). Other RCTs have concluded that flavonoid‐rich citrus juices improve cognitive functions in healthy older adults (n = 37) after 8 weeks of intake (Kean et al., 2015) and in healthy middle‐aged adults (n = 22) after acute administration (Alharbi et al., 2016). However, although cerebral blood flow was increased after acute intake by healthy young adults (n = 16), effects on cognitive functions (n = 24) were not observed (Lamport et al., 2016). These studies did determine the concentrations of some flavonoids in the citrus juices tested, although the complete chemical profiles were not evaluated.
The contrasting study outcomes may be due to variables such as study design; however, variation in chemical compositions of nutraceuticals such as those from citrus fruits also need to be considered, emphasising the importance of more extensive chemical characterisation of such products. In summary, there is emerging evidence for the potential use of certain berry and citrus fruits in the prevention or amelioration of cognitive decline; however, further investigations on the pharmacokinetics of specific constituents, clinical relevance, and mechanisms of action are required.
2.4. Tea, coffee, and cocoa
Green tea (Camellia sinensis) leaves contain polyphenols, with catechins as the major constituents. It is also important to consider that catechins occur in other foods/nutraceuticals including grapes (see above) and cocoa (see below). Epidemiological studies associate regular green tea intake with reduced age‐related cognitive decline and suggest that tea drinking reduces dementia risk (Williams et al., 2011). Many studies have therefore focused on the tea catechins to ascertain the pharmacological basis for these findings, with a particular focus on https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7002 (EGCG).
Much research has focused on the antioxidant mechanisms of catechins, which are scavengers of ROS and metal ion chelators, with EGCG showing more potent effects due to the trihydroxyl group on the B ring and the gallate moiety at the 3′ position in the C ring (Williams et al., 2011). These antioxidant mechanisms have been translated to more specific studies relevant to cognitive functions and dementia. EGCG scavenges ROS to protect hippocampal neurons against Aβ in vitro and in vivo, with the latter associated with reduced hippocampal lipid peroxide and oxidative stress; other mechanisms of EGCG that may protect neurons against oxidative stress are described in Table 1. In addition to reducing oxidative stress in an animal model of AD, EGCG also inhibited https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2465 (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2465) (Howes & Simmonds, 2014), indicating multiple mechanistic effects relevant to neuroprotection and cholinergic function. EGCG is anti‐inflammatory via different mechanisms and inhibits the neuroinflammatory response of microglia to Aβ (Cascella, Bimonte, Muzio, Schiavone, & Cuomo, 2017). With respect to AD pathology, EGCG is also a potent inhibitor of β‐ and γ‐secretase activities, with the pyrogalloyl moiety considered essential for activity to prevent Aβ aggregation and to reduce plaque load, effects that improve cognitive functions in vivo (Costa et al., 2017; Williams et al., 2011).
Some small RCTs conclude that although EGCG can modulate cerebral blood flow and electroencephalographic activity, it does not significantly improve cognitive functions; yet in contrast, a separate RCT concluded that green tea intake (2 g daily for 3 months) could improve cognitive performance (Cascella et al., 2017), suggesting that EGCG is not the only active constituent and that other green tea chemicals are important to modulate cognitive functions. Indeed, in vivo studies show tea catechins to improve memory acquisition and retention in SAM and to suppress oxidative damage to DNA in SAM brain, while epicatechin increases GSH levels in astrocytes, and it improves memory by ameliorating lipid peroxidation and ROS in rats with Aβ‐induced hippocampal toxicity (Williams et al., 2011).
Cocoa (Theobroma cacao) beans are often processed to make chocolate or other food products. Like tea, cocoa also contains catechins, which are classed as flavan‐3‐ol monomers (flavanols), in addition to various oligomers of these constituents, the proanthocyanidins. They have potent antioxidant effects via different mechanisms, in addition to anti‐inflammatory activity, and can influence vascular function (Ioannine et al., 2015). Processing of cocoa beans has been shown to influence the chemical profiles and antioxidant activity of the resulting cocoa products (Ioannine et al., 2015), which introduces complexities of variation and issues with standardisation, when they are assessed for their effects on cognition. Pharmacologically, cocoa flavanols promote neurogenesis, neuronal function and brain connectivity and they improve angiogenesis and cerebral blood flow in the brain (Dominguez & Barbagallo, 2018), with the latter effect observed in healthy older adults in a controlled trial (Lamport et al., 2015). Although the antioxidant and other effects of cocoa flavanols, including enhanced expression of neuroprotective and neuromodulatory proteins (Sokolov, Pavlova, Klosterhalfen, & Enck, 2013), are relevant to brain health and cognition in the long term, it is the vascular mechanistic actions of cocoa flavanols that are considered to largely explain their effects on cognitive functions.
Cocoa flavanols have been investigated in various RCTs and prospective studies. One study in the latter group concluded that chocolate intake for 2 years was associated with a lower risk of cognitive decline (41%) in 531 persons aged ≥65 years (Dominguez & Barbagallo, 2018). In a longitudinal study, habitual chocolate intake by individuals (age 23–98 years; n = 968) was associated with better cognitive performance when assessed by different neuropsychological tests and statistically controlled for cardiovascular, lifestyle and dietary factors (with the exception of working memory; Crichton, Elias, & Alkerwi, 2016). Small RCTs have shown enhanced denate gyrus function in healthy 50‐ to 69‐year‐olds, measured by cognitive tests after a cocoa flavanol containing diet for 3 months; increased brain perfusion in subjects (age 50–65 years) after intake of 494 mg of cocoa flavanols; and increased blood flow velocity in the middle cerebral artery after 1 and 2 weeks of cocoa flavanol intake by healthy subjects (mean age 72 years; Dominguez & Barbagallo, 2018). Other RCTs also provide supportive data to conclude benefits of cocoa flavanols on cognitive functions, including in healthy young adults and in elderly people with MCI (Massee et al., 2015; Sokolov et al., 2013). However, not all studies have concluded such effects, with a flavonoid‐rich diet (in placebo groups) suggested to explain the lack of efficacy in some RCTs (Scholey & Owen, 2013). Many studies (laboratory and clinically) investigating the effects of cocoa flavanols do not define the specific chemical profile or identities of the cocoa flavanols studied. Variation in such cocoa flavanol profiles (qualitatively and quantitatively) may explain conflicting results with respect to their effects on cognitive functions. In this context, the detailed chemical profiles of all tested natural product mixtures, including the cocoa flavanols, should be determined for conclusions from study outcomes to be more robust and directly comparative.
The effects of cocoa flavanols on cardiovascular health are widely documented, and age‐related dysfunction of cerebral blood flow may be accompanied by cognitive decline (Sokolov et al., 2013). Furthermore, there are known vascular risk factors for vascular dementia and cardiovascular risk factors linked with AD risk (Howes & Perry, 2011). Therefore, cocoa flavanols may mediate indirect effects on cognitive functions with ageing, through effects on cardiovascular or cerebrovascular health, although the calorific, fat and sugar content of certain cocoa products should also be considered in this respect.
Like tea and coffee, cocoa is a source of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=407, which has known effects on CNS function (see below). Cocoa also contains the related xanthine alkaloid, theobromine, which has been suggested to have a role in long‐term neuroprotection against neurotoxicity and to improve cerebral blood flow, but there is a lack of evidence for its ability to modulate cognitive functions (Cova, Leta, Mariani, Pantoni, & Pomati, 2019). Caffeine occurs at high concentrations in coffee (Coffea species), which is an https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=19 receptor antagonist, and it inhibits glutamate release for neuroprotection; caffeine also reduces Aβ (Howes, 2013) and so appears to act via different mechanisms to preserve or improve cognitive functions. Other studies show that caffeine has multiple mechanistic effects relevant to cognitive functions and AD (Table 1). Caffeine also protects against BBB disruptions in animal models of AD and PD, which may protect the CNS, particularly since BBB dysfunction has been implicated in the pathogenesis of neurodegenerative diseases that lead to cognitive decline (Howes, 2013).
Although most studies have focused on caffeine, other coffee constituents may also contribute to its use in the diet or as nutraceuticals, to influence cognitive functions. Trigonelline, a pyridine alkaloid in coffee, increases neurite outgrowth and inhibits AChE in vitro, and it reduces oxidative stress, astrocyte activity and inflammation to protect against Aβ and to improve memory in an animal model of AD (Fahanik‐Babaei, Baluchnejadmojarad, Nikbakht, & Roghani, 2019; Howes, 2013). The principal coffee polyphenols are isomers of caffeoylquinic acid including chlorogenic acid, which also mediates effects relevant to AD (Table 1). Caffeoyl‐, dicaffeoyl‐ and feruloyl‐quinic acids from coffee improved some cognitive functions in healthy participants (n = 38) when taken for 16 weeks in an RCT (Saitou et al., 2018). A similar combination of these polyphenols also improved some memory functions in elderly subjects with cognitive decline (n = 6) when taken for 6 months in a small pilot study (Kato, Ochiai, Kozuma, Sato, & Katsuragi, 2018). A minor coffee component is eicosanoyl‐5‐hydroxytryptamide and it ameliorated cognitive impairment and tau hyperphosphorylation in an animal model of AD, when taken orally for 6–12 months (Howes & Simmonds, 2014). In conclusion, these different chemical classes may contribute collectively to the effects of coffee in the diet or as a nutraceutical against cognitive decline.
Some epidemiological data indicate that coffee or caffeine intake may preserve cognitive functions with ageing. Indeed, studies suggest that dementia risk is reduced in coffee drinkers, with one study reporting a 65% reduction in risk, compared with no or low coffee intake, although not all studies are in agreement with this conclusion (Howes, 2013; Larsson & Orsini, 2018; Liu et al., 2016; Perry & Howes, 2011; Wu, Sun, & He, 2017). Other studies have associated high serum caffeine levels in older persons with MCI, with reduced progression to dementia (Dominguez & Barbagallo, 2018). Yet in a recent large‐scale Mendelian randomisation study that included over 300,000 coffee drinkers, there was no evidence that coffee intake could beneficially affect global cognition or memory (Zhou et al., 2018). Many factors may influence study results providing conflicting risk outcomes, including effects of diet and lifestyle, the frequency of coffee intake and the type of coffee (species of Coffea) ingested, and non‐standardised coffee chemical profiles. Indeed, one study has suggested that robusta coffee (Coffea canephora) and arabica coffee (Coffea arabica) may have different effects on memory and attention (Alharbi, Azmat, & Ahmed, 2018) and bean processing methods may also affect their chemistry (Wongsa, Khampa, Horadee, Chaiwarith, & Rattanapanone, 2019) and therefore biological activities.
2.5. Saffron
Saffron consists of the stigmas of Crocus sativus and is widely used for culinary applications, although it has been used medicinally for its reputed effects on the CNS (Howes & Houghton, 2012). There has been much interest in saffron as a nutraceutical to reduce cognitive decline in ageing or in age‐related diseases, especially dementia. In different animal models of cognitive impairment, saffron extracts attenuate memory deficits (Bukhari, Manzoor, & Dhar, 2018; Christodoulou, Kadoglou, Kostomitsopoulos, & Valsami, 2015).
There is a lack of clinical data to determine the effects of saffron on cognitive functions in dementia patients or in the general ageing population and those with MCI, as only a few clinical studies have been conducted to date. RCTs show that saffron extracts (30 mg daily for 16 or 22 weeks) can improve cognitive functions in AD patients when compared with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6599 (AChE inhibitor drug) with fewer adverse effects; the same dose taken for 1 year was comparable with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4253 (NMDA receptor antagonist) in reducing cognitive decline in AD patients (Howes, Fang, & Houghton, 2017). In a small study (n = 35), patients with MCI were suggested to have improved cognitive functions after 1 year of saffron supplementation (Tsolaki et al., 2016), although the study design and supplement details were not clearly defined. Saffron combined with known cognition‐enhancing plants (the herbal medicines Ginkgo biloba and Panax ginseng), to give the formula known as Sailuotong, is suggested to improve cognitive symptoms in vascular dementia and is currently being evaluated in an RCT to establish any evidence for efficacy in older adults with MCI (Steiner, Bensoussan, Liu, Hohenberg, & Chang, 2018).
The chemical constituents responsible for these observed effects have not been fully elucidated. Saffron contains monoterpenes, such as safranal and flavonols derived from kaempferol, in addition to crocins, which are in the carotenoid class of phytochemicals (Christodoulou et al., 2015). Most studies have focused on the crocins (a series of different sugar esters of the dicarboxylic acid crocetin), to explain the cognitive‐enhancing effects of saffron, although the clinical relevance of mechanistic studies requires further evaluation. This is because many studies report the effects of crocins that are not defined in composition or chemical structure. Furthermore, in vivo, crocins have poor intestinal absorption, and they are hydrolysed to crocetin in the gut (Howes et al., 2017); thus, the effects of crocetin would be more useful to evaluate mechanistically. One study has shown trans‐crocetin to pass through the blood–CSF barrier and to penetrate Caco‐2 monolayer cells more effectively compared with crocin‐1, while crocetin is rapidly absorbed and can be detected in plasma after oral administration to humans; furthermore, trans‐crocetin can cross the BBB (Hashemi & Hosseinzadeh, 2019).
With respect to dementia, crocin inhibits Aβ formation and aggregation, with trans‐crocin‐4 in particular inhibiting Aβ fibrillogenesis (Howes et al., 2017; Howes & Simmonds, 2014). A recent study evaluated IIIM‐141, a standardised mixture of crocins containing trans‐4‐GC‐crocin (36% w/w) and concluded it could improve cognitive functions in different animal models of memory impairment and could induce P‐gp and so may enhance Aβ clearance from the brain; it also protects against the neurotoxic effects of both Aβ and glutamate in vitro (Bharate et al., 2018). Indeed, another study revealed that in an animal model of PD, crocin was suggested to be neuroprotective via NMDA receptor modulation (Howes & Houghton, 2012). A different saffron constituent, safranal, could reduce the kainic acid‐induced increase of extracellular glutamate concentrations in the rat hippocampus (Pitsikas, 2015), suggesting that modulation of glutamatergic function by both safranal and crocin (or metabolites) may contribute to the cognitive effects of saffron. Other studies suggest that crocin is neuroprotective via antioxidant and anti‐inflammatory mechanisms (Howes & Houghton, 2012). In cultured microglia, both crocin and crocetin inhibited https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2509 release and reduced TNF‐α, IL‐1β, and intracellular ROS, in addition to inhibiting hippocampal cell death (Moshiri, Vahabzadeh, & Hosseinzadeh, 2015). Reduction of pro‐inflammatory cytokines in addition to anti‐apoptotic effects was concluded to explain the ability of crocetin to reverse Aβ‐induced memory deficits in an animal model of AD (Zhang, Wang, Dong, & Liu, 2018). Safranal is another constituent that protects against Aβ‐ and H2O2‐induced toxicity in vitro (PC12 cells) and the reduction in apoptosis was attributed to modulation of the MAPK and P13K pathways (Rafieipour, Hadipour, Emami, Asili, & Tayarani‐Najaran, 2019). Crocetin also enhanced antioxidant enzymes, including GSH reductase and SOD, and reduced lipid peroxidation to mediate neuroprotective effects in an animal model of PD (Hashemi & Hosseinzadeh, 2019) suggesting the ability to cross the BBB, with potential to mediate neuroprotection in cognitive disorders. The carotenoid classes of constituents from saffron can also form complex ligand–polynucleotides that may protect DNA and RNA (Leone et al., 2018), relevant to neuroprotection in the CNS.
Inhibition of AChE is suggested to be another mechanism to mediate the cognitive‐enhancing effects of saffron, although one study reported that inhibition by an extract is only by 30% (Bukhari et al., 2018; Pitsikas, 2015), so this mechanism alone may not significantly contribute to the effects of saffron on cognitive functions. Yet a separate in vivo study reported that saffron treatment (ad libitum) in mice protected against cognitive decline induced by aflatoxin B1 and significantly reduced AChE activity in the brain (Linardaki, Lamari, & Margarity, 2017), indicating that modulation of cholinergic function may contribute at least partly to the effects of saffron on cognition. Saffron extracts and constituents have also shown mechanistic effects relevant to glucose control and diabetes and to ischaemic heart disease (Christodoulou et al., 2015; Leone et al., 2018); as these diseases are considered to be risk factors for cognitive decline and dementia, saffron may mediate indirect effects on cognitive functions with ageing by mechanistic effects relevant to these other diseases.
2.6. Plants with omega fatty acids
Dietary fatty acids can be classified as saturated, monounsaturated (MUFA), or polyunsaturated (PUFA), depending on the number of double bonds in their carbon chains. PUFAs include omega‐3 (ω‐3 or n‐3) and omega‐6 (ω‐6 or n‐6); ω‐3 PUFAs include https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1049, eicopentaenoic acid and docosahexaenoic acid (DHA),while ω‐6 PUFAs include https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1052 and arachidonic acid (ARA). In human diets, the main source of eicopentaenoic acid and DHA is fish, while α‐linolenic acid is available in oils from seeds and fruits including flax (Linum usitatissimum), walnuts (Juglans species), peanuts (Arachis hypogaea), olives (Olea europaea) and black cumin (Nigella sativa) among other species (Abedi & Sahari, 2014). Linolenic acid is obtained from sunflower (Helianthus species), corn (Zea mays), sesame (Sesamum indicum) and almonds (Prunus dulcis); ARA is found in meat, eggs and dairy products (Kaur, Chugh, & Gupta, 2014). Given that ω‐6 PUFAs predominate over ω‐3 PUFAs in most Western diets, a pro‐inflammatory environment is elicited by an amplified production of ARA‐derived PGs and leukotrienes (Calder, 2013), which is relevant to disease states including those influencing cognitive functions (Howes, 2018).
Prospective studies show that high intakes of PUFAs and MUFAs can prevent cognitive decline in the elderly, while cohort studies indicate that the consumption of ω‐3 PUFAs decreases the risk of AD (Gillette‐Guyonnet, Secher, & Vellas, 2013). Clinical studies have focused on the effects of PUFAs derived from fish oils or DHA, with a number reporting cognitive benefits in healthy adults, healthy older adults and patients with mild cognitive dysfunction in AD (Freund‐Levi et al., 2006; Witte et al., 2013). Although numerous plant sources of PUFAs and MUFAs are in the human diet and are of interest as nutraceuticals, there have been comparatively fewer studies to determine their effects on cognitive functions or on dementia risk. A Cochrane review in 2016 evaluated whether ω‐3 PUFAs from both plant and fish sources had any benefits in dementia patients, but there was no evidence for efficacy in the treatment of mild‐to‐moderate AD (Burckhardt et al., 2016).
In contrast to the lack of evidence for efficacy in dementia, epidemiological studies associate a Mediterranean diet (characterised by high intakes of vegetables, fruits, nuts, legumes, cereals, herbs, and oils such as olive‐derived containing PUFAs) with a lower overall mortality from certain diseases, including cardiovascular (a risk factor for dementia), and with a possible reduced risk of AD; evidence also suggests that a high intake of PUFAs, such as those in olive oil, may protect against cognitive decline, although the level of any protective effect remains unclear (Perry & Howes, 2011). A prospective study involving 3,831 (age ≥ 65 years) concluded that Mediterranean dietary patterns were associated with consistently higher levels of cognitive functions over 11 years and it was suggested that whole grains, legumes and nuts may be neuroprotective and were positively correlated with higher cognitive functions (Wengreen et al., 2013). Nuts are a source of PUFAs that may have contributed to these observed benefits. It therefore appears that PUFAs may contribute to maintaining cognitive functions with ageing, but more research is needed to evaluate the effects of specific plant oils and their PUFA constituents, via the diet and as nutraceuticals.
2.7. Herbs and spices relevant to cognition
Herbs in the mint family (Lamiaceae) are mainly used today for flavouring food or as teas. Since the discovery that aromatic European sages (Salvia officinalis and S. officinalis subsp. lavandulifolia; synonym for the latter: S. lavandulifolia) and individual constituents inhibit AChE and have a range of other bioactivities relevant to cognition (Howes & Houghton, 2012). Studies on their phytochemicals, to maintain, enhance and protect cognition have increased, warranting placement of these plants as key nutraceuticals for cognition. Aromatic edible plants in the mint family, used in traditional medicine through history for cognitive benefits and tested in clinical trials for cognitive effects, include sage (S. officinalis and S. officinalis subsp. lavandulifolia;), rosemary (Salvia rosmarinus; synonym: Rosmarinus officinalis), lemon balm (Melissa officinalis) and mints (Mentha species (Perry et al., 2018; Perry & Howes, 2011).
Distinct neuroactive phytochemicals are common to these plants and include terpenes and flavonoids and their glycosides. A number of Lamiaceae phytochemicals, for example, 1,8‐cineole and rosmarinic acid, are active in a range of in vitro and in vivo studies related to cognitive function (Table 1). Numerous plant monoterpenes, sesquiterpenes and diterpenes from plant sources have shown the ability to inhibit AChE (Williams et al., 2011), while various plant essential oils have shown different mechanistic effects relevant to anti‐ageing, neuroprotection and AD, as reviewed previously (Benny & Thomas, 2019). Certain Lamiaceae diterpenes are also associated with other mechanistic effects relevant to cognitive functions and dementia; for example, carnosic acid that occurs in sage and rosemary modulates neurite outgrowth (Howes & Perry, 2011), although the clinical significance of these actions requires evaluation. Other phytochemicals in aromatic herbs and spices such as black cumin (N. sativa) include the monoterpene thymoquinone, are also being researched their effects on health, including cognition (see below and Table 1).
Lamiaceae plants with centuries‐old reputations for enhancing memory have been studied in RCTs and shown to enhance cognition (memory and alertness) in healthy, young and older people and to counter cognitive impairment as well as improve behavioural measures in people with AD (Perry et al., 2017) and to have positive effects on blood lipid (lowering cholesterol) and antioxidant profiles in RCTs, both of which are relevant to cognition. Rosmarinic acid, which occurs in the sub‐family Nepetoidae, has shown numerous mechanistic effects relevant to cognition and AD, as reviewed previously (Williams et al., 2011) and summarised in Table 1, although its ability to cross the BBB may be limited (Perry et al., 2018). It is interesting to note that spearmint (Mentha spicata) is reported to contain the highest concentration (58%) of rosmarinic acid, followed closely by sage (S. officinalis; 37%) and rosemary (7%; Shekarchi, Hajimehdipoor, Saeidnia, Gohari, & Hamedani, 2012).
Containing a number of the same key phytochemicals as S. officinalis (1,8‐cineole, borneol and rosmarinic acid) is rosemary and in clinical studies, extracts improve attention and memory in healthy students, adults and the elderly (Nematolahi, Mehrabani, Karami‐Mohajeri, & Dabaghzadeh, 2018). Both in vitro and in vivo, rosemary inhibits AChE, activates https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=76 s, enhances blood capillary flow, improves cognitive deficits, is anti‐Aβ, neuroprotective, antioxidant, anti‐inflammatory and extends life span in fruit flies (Andrade et al., 2018; Wake et al., 2000). In RCTs, peppermint (Mentha × piperita) and spearmint (M. spicata) extracts/oils also improve cognitive functions (Table 1). One study concluded that a peppermint oil, characterised by high concentrations of the monoterpenes https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2430 and menthone, has in vitro AChE inhibitory, calcium regulator, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=72/nicotinic receptor‐binding properties and improved performance in demanding cognitive tasks, as well as attenuating the increase in mental fatigue associated with extended cognitive task performance in healthy adults (Kennedy et al., 2018). Lemon balm, used as a culinary herb, improved cognitive functions in controlled trials (Table 1) and showed cardiovascular and antidiabetic benefits in one RCT—both relevant to maintaining cognition (Asadi et al., 2018).
The Lamiaceae volatile (essential) oils have been widely studied for effects on mood and cognition. Monoterpenes, and their derivatives, are the major constituents of many volatile oils and of growing interest as neuroactive phytochemicals, since they are lipophilic and of low MW and thus may be readily absorbed through the skin and cross into the brain. Monoterpenes have been shown as neuroactive in a range of studies relevant to cognition and AD (Okello & Howes, 2018; also see Table 1). Two monoterpenes (used in mainstream medicine for topical analgesia) are https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2430 (Lai, Collaku, & Reed, 2017) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6413 (the latter occurs in cinnamon [Cinnamomum species] resin at 96%), which act via the transient receptor potential melenastatin (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=500; Wang et al., 2017). The clinical improvements in memory seen by Lamiaceae species are likely due to the polypharmacology of constituents (referred to above). Distinct monoterpene components can bind to more than one site on the AChE enzyme and one component can favour the interaction of a second (Jankowska et al., 2017) giving synergistic effects (Wake et al., 2000). Other complex interactions include thujone, which inhibits AChE yet impairs memory in a laboratory model by inhibiting ACh by interfering with nicotinic receptors, whereas camphor and borneol also present in sage and lemon balm activate nicotinic receptors (Wake et al., 2000).
1,8‐Cineole occurs in sage (S. officinalis 40–50%), Spanish sage (S. officinalis subsp. lavandulifolia 20%), and rosemary (20%) and has been studied for its potential role in a range of chronic diseases, including those relevant to cognition (Table 1). It has been tested for clinical efficacy in anxiety and shown to cross the BBB, including via inhalation, with 1,8‐cineole blood levels being correlated with increased cognitive performance (Okello & Howes, 2018). Other plant foodstuffs, used for their cognitive benefits in traditional medicine and therefore potential key cognition nutraceuticals, supported by some RCT evidence include the spice black cumin (N. sativa), which is also a source of PUFAs (see above). Black cumin enhances cognition, verbal learning, memory and attention in the elderly (Bin Sayeed et al., 2014), along with anti‐AChE, glutamatergic, antioxidant, anti‐inflammatory and neuroprotective effects (Samarghandian, Farkhondeh, & Samini, 2018). It contains alkaloids including nigellicine, saponins and the monoterpenes carvacrol and thymol which have nicotinic activity, pinenes with anti‐AChE effects and thymoquinone, which has been significantly studied for therapeutic benefits with a focus on neuroprotective effects, for example as a https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=468 agonist (Darakhshan, Bidmeshki Pour, Hosseinzadeh Colagar, & Sisakhtnezhad, 2015; Ibrahim, Zickri, Aal, Heikal, & Osama, 2016).
Bacopa (Bacopa monnieri) is a plant food used in salads and dahls, and as an Ayurvedic medicine used for memory. In an RCT meta‐analysis, it improved cognition, attention and memory in child, adolescent, adult, and elderly populations and improved memory in multi‐task stress activities and in pilot trials for mild cognitive impairment (Downey et al., 2013; Howes & Houghton, 2012; Kongkeaw, Dilokthornsakul, Thanarangsarit, Limpeanchob, & Norman, 2014). Bacopa improves memory in several laboratory models, inhibits AChE, is neuroprotective, promotes neurogenesis, increases blood flow, is also anti‐inflammatory and antioxidant, and increases longevity in tests mimicking ageing (Howes & Houghton, 2012). Pine (Pinus roxburghii), the resin of which used to make the drink retsina, improves memory in laboratory models, while Monterey pine (Pinus radiata) improves cognitive function in brain injury and increases the speed of memory in a pilot double‐blind trial (Pipingas et al., 2008). There are a number of other dietary plant species of interest as nutraceuticals that have been studied pharmacologically and/or in preliminary clinical trials to demonstrate their cognitive benefits. These include gotu kola (Centella asiatica), a green leafy vegetable (Howes & Houghton, 2012), lemon verbena (Aloysia citrodora; Abuhamdah et al., 2015), asparagus (Asparagus species; Pahwa & Goel, 2016), pomegranate (Punica granatum; Subash et al., 2015) and holy basil (Ocimum tenuiflorum; Giridharan et al., 2011).
3. STUDY APPROACHES: PAST, PRESENT, AND FUTURE
Above and in Table 1, we have reviewed the evidence for the role of certain dietary phytochemicals as nutraceuticals relevant to cognitive decline in ageing. As discussed above (e.g. turmeric, grapes/red wine, cocoa and coffee), the mechanistic data for dietary plants and their constituents are often more promising, when compared with outcomes from clinical studies. Indeed, positive RCT evidence for nutraceuticals in general (across the broader spectrum of supplements including vitamins and fish‐derived PUFAs) is limited, as concluded in a review that appraised evidence from RCTs, systematic reviews, meta‐analyses and observation studies (Rakesh et al., 2017). Furthermore, prospective studies on phytochemicals as nutraceuticals are limited and outcomes are often variable (as discussed above for coffee and wine intake). One of the major challenges with interpreting such prospective studies is the considerable variation that can occur in the chemical profiles of the dietary plants evaluated. Most studies have not botanically or chemically defined the plant product. Thus the chemical variation (qualitative and quantitative) introduces additional variables and challenges, against the backdrop of other factors such as intake frequency, concentration and dose. Above, we discuss how different species of Coffea have different chemistry and pharmacological activities, which would clearly impact on outcomes for both clinical and prospective studies. We therefore suggest that future studies capture more specific details of the dietary/nutraceutical intervention, such that outcomes are more informative and studies can be compared more directly.
Above, we also discuss the mechanistic effects of phytochemical nutraceuticals that are known to be associated with cognitive decline (normal and pathological) in ageing, with targets including protein aggregation, inflammation, oxidation and cholinergic function. Indeed, so far, only cholinergic therapy and NMDA receptor modulation have provided any benefit therapeutically, justifying a focus on these systems. Future studies on phytochemical nutraceuticals should also encompass emerging knowledge on the mechanisms involved in cognitive decline and evidence for potential factors that may contribute to the onset of cognitive deficits in dementia. For example, above we discuss nutraceuticals (e.g. cocoa flavanols) that may influence cognitive decline by ameliorating risk factors (e.g. cardiovascular) for dementia. Furthermore, the bacteria Porphyromonas gingivalis and the toxic proteases (gingipains) they produce are involved in the pathogenesis of periodontitis but have recently been found to occur in the brains of AD patients (Dominy et al., 2019), raising the suggestion that interventions that target this bacterium may be a future approach to evaluate with respect to AD risk.
4. CONCLUSION
To judge by the increasing volume of research (particularly laboratory based), there is no shortage of promising nutritional interventions involving plant foods and their phytochemicals to protect or counter cognitive decline in ageing. Animal models and in vitro studies indicate new directions and/or back up human studies. However, no animal model (meeting current ethical approval) has the cognitive capacity or prolonged ageing of man. Controlled human intervention studies are therefore key, though challenging, particularly in relation to protection.
According to a recent meta‐analysis of controlled nutritional interventions for cognition (Solfizzi et al., 2018), “Antioxidant‐rich foods (nuts, grapes, cherries) and fatty acid supplementation, mainly n‐3 polyunsaturated fatty acids (PUFA), improved specific cognitive domains and cognitive‐related outcomes in MCI, mild‐to‐moderate dementia, and AD.” Since then, meta‐analyses of other promising plant foods such as turmeric, grapes (red wine) and coffee have been inconclusive.
Dietary intervention studies of cognitive function in the aged face challenges of diagnosis, dose, standardisation, placebo control, blinding (for the subject) and subject individuality in terms of genetics and life experience. Added to this complexity are potential effects of phyto‐nutraceuticals on brain functions other than cognition, which in turn affect cognition (sleep or mood, for example), and on other systems that affect brain function (cardiovascular or blood glucose regulation, for example). Some of these challenges are resolved for individual phytochemicals, ideally where a specific neuropharmacological mechanism is identified. The phytochemicals discussed in this review are not brain specific in their pharmacological actions and costs of taking a single chemical as opposed to whole plant food are high and not supported by health care providers. It is apparent that pharmacological and clinical research on nutraceuticals relevant to cognition is often more focused on the concept of the “single active ingredient,” with examples including curcumin and resveratrol. In this review, we have discussed the inconclusive findings that are often observed when such single constituents are tested for their effects on cognitive functions in clinical studies. We have also discussed the epidemiological studies that associate certain dietary interventions with a reduced risk of cognitive decline in ageing or pathological dementia; such examples include red wine and coffee. It is in this context that we highlight that many laboratory or clinical studies on single chemicals are very different, in terms of dose (concentration and frequency), study duration and potential for adverse effects and chemical composition, compared with the highly complex, multi‐component and variable in composition of phytochemicals that are taken in the diet or in the form of mixtures as nutraceuticals. Although this review is focused on certain “single active ingredients,” this is because most studies have focused on these, which can be more easily sourced (commercially) and investigated. We emphasise the need to investigate the many other phytochemicals in promising dietary or nutraceutical plants, to provide more comprehensive evidence for the role of plants to modulate cognitive decline and not just their major constituents that are more widely available. This is against a backdrop of challenges of the chemical variation and standardisation of plant foods, which introduces additional complexities for interpreting studies to evaluate their effects on cognitive functions.
The chemical structures of phytochemicals with effects relevant to cognitive functions are diverse, ranging from alkaloids to different sub‐classes of flavonoids and terpenoids, among other chemical classes; examples of this chemical diversity are presented in Figure 1. Since these different chemicals are likely relevant to different types of age‐related cognitive conditions, multiple effects of food plants containing a range of neuroactive phytochemicals could be ideal for protection. “Polypharmacological” diets could be designed to incorporate plant foods rich in a range of these chemicals.
Figure 1.
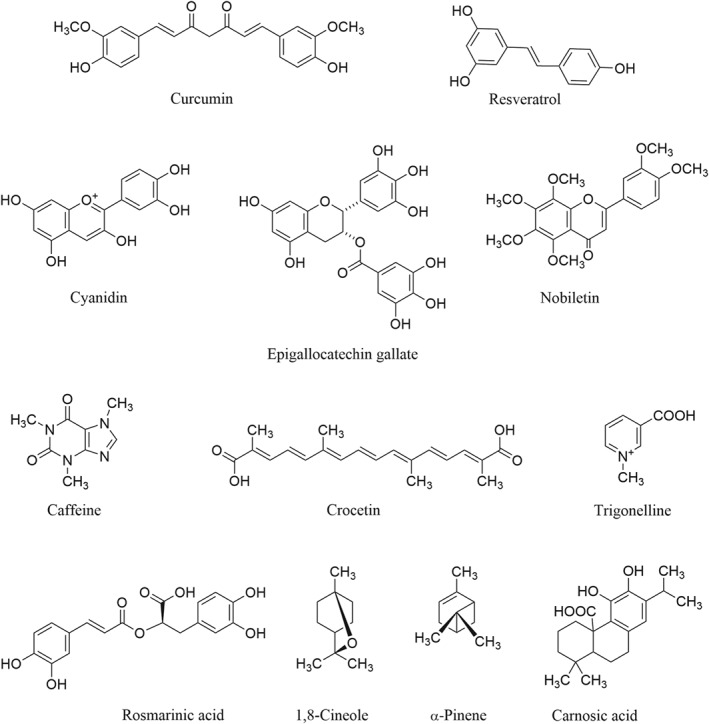
Dietary and nutraceutical plant chemicals relevant to cognitive functions are diverse in structure; examples are shown that occur in turmeric (curcumin), grapes and red wine (resveratrol), blueberries (cyanidin), tea (epigallocatechin gallate), citrus fruit (nobiletin), coffee (caffeine and trigonelline), saffron (crocetin) and sage and rosemary (rosmarinic acid, carnosic acid, α‐pinene, and 1,8‐cineole)
Another advantage of research into whole plant foods as opposed to plant chemicals for cognitive benefit is that there will be as yet unidentified neuroactive phytochemicals to be discovered. Discovering new food plants or nutraceuticals with cognitive benefits could also lead to novel insights into mechanisms of, for example, information storage or retrieval. A classic neuro‐phyto‐pharmacological example is the discovery from the opium poppy (Papaver somniferum) not only of the most powerful plant analgesic (morphine) but also of pain control mechanisms involving endogenous opiates (enkephalins and endorphins) and their opioid receptors.
Pharmacological approaches to preventing or treating human brain dysfunction, or enhancing cognitive function, are no doubt best combined with psychological or physiological approaches. The clarion call “Diet and Exercise” cannot perhaps be heard too early in life!
4.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Howes M‐JR, Perry NSL, Vásquez‐Londoño C, Perry EK. Role of phytochemicals as nutraceuticals for cognitive functions affected in ageing. Br J Pharmacol. 2020;177:1294–1315. 10.1111/bph.14898
This article is published with the permission of the Controller of HMSO and the Queen's Printer for Scotland.
REFERENCES
- Abedi, E. , & Sahari, M. A. (2014). Long‐chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Science & Nutrition, 2, 443–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuhamdah, S. , Abuhamdah, R. , Howes, M.‐J. , Al‐Olimat, S. , Ennaceur, A. , & Chazot, P. (2015). Pharmacological and neuroprotective profile of an essential oil derived from leaves of Aloysia citrodora Palau. Journal of Pharmacy and Pharmacology, 67, 1306–1315. [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E.,. L. , Striessnig, J. , KeLly, E. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2017/18: ion channels. British Journal of Pharmacology, 176, S142–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi, M. H. , Lamport, D. J. , Dodd, G. F. , Saunders, C. , Harkness, L. , Butler, L. T. , & Spencer, J. P. E. (2016). Flavonoid‐rich orange juice is associated with acute improvements in cognitive function in healthy middle‐aged males. European Journal of Nutrition, 55, 2021–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi, W. D. M. , Azmat, A. , & Ahmed, M. (2018). Comparative effect of coffee robusta and coffee arabica (Qahwa) on memory and attention. Metabolic Brain Disease, 33, 1203–1210. [DOI] [PubMed] [Google Scholar]
- Andrade, J. M. , Faustino, C. , Garcia, C. , Ladeiras, D. , Reis, C. P. , & Rijo, P. (2018). Rosmarinus officinalis L. An update review of its phytochemistry and biological activity. Future Science, 4, FSO283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres‐Lacueva, C. , Shukitt‐Hale, B. , Galli, R. L. , Jauregui, O. , Lamuela‐Raventos, R. M. , & Joseph, J. A. (2005). Anthocyanins in aged blueberry‐fed rats are found centrally and may enhance memory. Nutritional Neuroscience, 8, 111–120. [DOI] [PubMed] [Google Scholar]
- Asadi, A. , Shidfar, F. , Safari, M. , Malek, M. , Hosseini, A. F. , Rezazadeh, S. , … Hosseini, S. (2018). Safety and efficacy of Melissa officinalis (lemon balm) on ApoA‐I, Apo B, lipid ratio and ICAM‐1 in type 2 diabetes patients. A randomized, double‐blinded clinical trial. Complementary Therapies in Medicine, 40, 83–88. [DOI] [PubMed] [Google Scholar]
- Barros, D. , Amaral, O. B. , Izquierdo, I. , Geracitano, L. , Raseira, M. D. C. B. , Henriques, A. T. , & Ramirez, M. R. (2006). Behavioral and genoprotective effects of Vaccinium berries intake in mice. Pharmacology Biochemistry and Behavior, 84, 229–234. [DOI] [PubMed] [Google Scholar]
- Benny, A. , & Thomas, J. (2019). Essential oils as treatment strategy for Alzheimer's disease. Current and future perspectives. Planta Medica, 85, 239–248. [DOI] [PubMed] [Google Scholar]
- Bharate, S. S. , Kumar, V. , Singh, G. , Singh, A. , Gupta, M. , Singh, D. , … Bharate, S. B. (2018). Preclinical development of Crocus sativus‐based botanical lead IIIM‐141 for Alzheimer's disease: Chemical standardization, efficacy, formulation development, pharmacokinetics, and safety pharmacology. ACS Omega, 3, 9572–9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin Sayeed, M. S. , Shams, T. , Fahim Hossain, S. , Rahman, M. R. , Mostofa, A. , Fahim Kadir, M. , & Asaduzzaman, M. (2014). Nigella sativa L. seeds modulate mood, anxiety and cognition in healthy adolescent males. Journal of Ethnopharmacology, 152, 156–162. [DOI] [PubMed] [Google Scholar]
- Bowtell, J. L. , Aboo‐Bakkar, Z. , Conway, M. E. , Adlam, A. L. R. , & Fulford, J. (2017). Enhanced task‐related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Applied Physiology, Nutrition, and Metabolism, 42, 773–779. [DOI] [PubMed] [Google Scholar]
- Brem, A. K. , & Sensi, S. L. (2018). Towards combinatorial approaches for preserving cognitive fitness in aging. Trends in Neurosciences, 41, 885–897. [DOI] [PubMed] [Google Scholar]
- Bukhari, S. I. , Manzoor, M. , & Dhar, M. K. (2018). A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomedicine and Pharmacotherapy, 98, 733–745. [DOI] [PubMed] [Google Scholar]
- Burckhardt, M. , Herke, M. , Wustmann, T. , Watzke, S. , Langer, G. , & Fink, A. (2016). Omega‐3 fatty acids for the treatment of dementia. Cochrane Database of Systematic Reviews, 2016, CD009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder, P. C. (2013). Omega‐3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? British Journal of Clinical Pharmacology, 75, 645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella, M. , Bimonte, S. , Muzio, M. R. , Schiavone, V. , & Cuomo, A. (2017). The efficacy of epigallocatechin‐3‐gallate (green tea) in the treatment of Alzheimer's disease: An overview of pre‐clinical studies and translational perspectives in clinical practice. Infectious Agents and Cancer, 12, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou, E. , Kadoglou, N. P. , Kostomitsopoulos, N. , & Valsami, G. (2015). Saffron: A natural product with potential pharmaceutical applications. Journal of Pharmacy and Pharmacology, 67, 1634–1649. [DOI] [PubMed] [Google Scholar]
- Cicero, A. F. G. , Fogacci, F. , & Banach, M. (2018). Botanicals and phytochemicals active on cognitive decline: The clinical evidence. Pharmacological Research, 130, 204–212. [DOI] [PubMed] [Google Scholar]
- Costa, C. , Tsatsakis, A. , Mamoulakis, C. , Teodoro, M. , Briguglio, G. , Caruso, E. , … Fenga, C. (2017). Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food and Chemical Toxicology, 110, 286–299. [DOI] [PubMed] [Google Scholar]
- Cova, I. , Leta, V. , Mariani, C. , Pantoni, L. , & Pomati, S. (2019). Exploring cocoa properties: Is theobromine a cognitive modulator? Psychopharmacology, 236, 561–572. [DOI] [PubMed] [Google Scholar]
- Cox, K. H. M. , Pipingas, A. , & Scholey, A. B. (2015). Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. Journal of Psychopharmacology, 29, 642–651. [DOI] [PubMed] [Google Scholar]
- Crichton, G. E. , Elias, M. F. , & Alkerwi, A. (2016). Chocolate intake is associated with better cognitive function: The Maine‐Syracuse Longitudinal Study. Appetite, 100, 126–132. [DOI] [PubMed] [Google Scholar]
- D'Cunha, N. M. , McKune, A. J. , Panagiotakos, D. B. , Georgousopoulou, E. N. , Thomas, J. , Mellor, D. D. , & Naumovski, N. (2019). Evaluation of dietary and lifestyle changes as modifiers of S100β levels in Alzheimer's disease. Nutritional Neuroscience, 22, 1–18. [DOI] [PubMed] [Google Scholar]
- Darakhshan, S. , Bidmeshki Pour, A. , Hosseinzadeh Colagar, A. , & Sisakhtnezhad, S. (2015). Thymoquinone and its therapeutic potentials. Pharmacological Research, 95‐96, 138–158. [DOI] [PubMed] [Google Scholar]
- De Domenico, S. , & Giudetti, A.M. (2017) Nutraceutical intervention in ageing brain. Journal of Gerontology and Geriatrics, 65, 79–92. [Google Scholar]
- Dominguez, L. J. , & Barbagallo, M. (2018). Nutritional prevention of cognitive decline and dementia. Acta Biomed, 89, 276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy, S. S. , Lynch, C. , Ermini, F. , Benedyk, M. , Marczyk, A. , Konradi, A. , … Potempa, J. (2019). Porphyromonas gingivalis in Alzheimer's disease brains: Evidence for disease causation and treatment with small‐molecule inhibitors. Science Advances, 5, eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey, L. A. , Kean, J. , Nemeh, F. , Lau, A. , Poll, A. , Gregory, R. , … Stough, C. (2013). An acute, double‐blind, placebo‐controlled crossover study of 320 mg and 640 mg doses of a special extract of Bacopa monnieri (CDRI 08) on sustained cognitive performance. Phytotherapy Research, PTR, 27, 1407–1413. [DOI] [PubMed] [Google Scholar]
- Eliassen, C. F. , Reinvang, I. , Selnes, P. , Grambaite, R. , Fladby, T. , & Hessen, E. (2017). Biomarkers in subtypes of mild cognitive impairment and subjective cognitive decline. Brain and Behavior, 7, e00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, H. M. , Howe, P. R. C. , & Wong, R. H. X. (2017). Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post‐menopausal women; a 14‐week randomised placebo‐controlled intervention trial. Nutrients, 9, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahanik‐Babaei, J. , Baluchnejadmojarad, T. , Nikbakht, F. , & Roghani, M. (2019). Trigonelline protects hippocampus against intracerebral Aβ(1–40) as a model of Alzheimer's disease in the rat: Insights into underlying mechanisms. Metabolic Brain Disease, 34, 191–201. [DOI] [PubMed] [Google Scholar]
- Falcone, P. H. , Tribby, A. C. , Vogel, R. M. , Joy, J. M. , Moon, J. R. , Slayton, C. A. , … Herrlinger, K. A. (2018). Efficacy of a nootropic spearmint extract on reactive agility. A randomized, double‐blind, placebo‐controlled, parallel trial. Journal of the International Society of Sports Nutrition, 15, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria, A. , Pestana, D. , Teixeira, D. , Azevedo, J. , De Freitas, V. , Mateus, N. , & Calhau, C. (2010). Flavonoid transport across RBE4 cells: A blood–brain barrier model. Cellular & Molecular Biology Letters, 15, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, K. , van Lent, D. M. , Wolfsgruber, S. , Weinhold, L. , Kleineidam, L. , Bickel, H. , … Wagner, M. (2018). Prospective associations between single foods, Alzheimer's dementia and memory decline in the elderly. Nutrients, 10, 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund‐Levi, Y. , Eriksdotter‐Jönhagen, M. , Cederholm, T. , Basun, H. , Faxen‐Irving, G. , Garlind, A. , … Palmblad, J. (2006). ω‐3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: A randomized double‐blind trial. Archives of Neurology, 63, 1402–1408. [DOI] [PubMed] [Google Scholar]
- Ghosh, S. , Banerjee, S. , & Sil, P. C. (2015). The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food and Chemical Toxicology, 83, 111–124. [DOI] [PubMed] [Google Scholar]
- Gillette‐Guyonnet, S. , Secher, M. , & Vellas, B. (2013). Nutrition and neurodegeneration: Epidemiological evidence and challenges for future research. British Journal of Clinical Pharmacology, 75, 738–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginneken, V. (2017). Are there any biomarkers of aging? Biomarkers of the brain. Biomedical Journal of Scientific & Technical Research, 1(1), 2017. [Google Scholar]
- Giridharan, V. V. , Thandavarayan, R. A. , Mani, V. , Ashok Dundapa, T. , Watanabe, K. , & Konishi, T. (2011). Ocimum sanctum Linn. leaf extracts inhibit acetylcholinesterase and improve cognition in rats with experimentally induced dementia. Journal of Medicinal Food, 14, 912–919. [DOI] [PubMed] [Google Scholar]
- Goozee, K. G. , Shah, T. M. , Sohrabi, H. R. , Rainey‐Smith, S. R. , Brown, B. , Verdile, G. , & Martins, R. N. (2016). Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer's disease. British Journal of Nutrition, 115, 449–465. [DOI] [PubMed] [Google Scholar]
- Gutierres, J. M. , Carvalho, F. B. , Schetinger, M. R. , Rodrigues, M. V. , Schmatz, R. , Pimentel, V. C. , & Leal, C. (2012). Protective effects of anthocyanins on the ectonucleotidase activity in the impairment of memory induced by scopolamine in adult rats. Life Sciences, 91, 1221–1228. [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi, M. , & Hosseinzadeh, H. (2019). A comprehensive review on biological activities and toxicology of crocetin. Food and Chemical Toxicology, 130, 44–60. [DOI] [PubMed] [Google Scholar]
- Herrup, K. (2015). The case for rejecting the amyloid cascade hypothesis. Nature Neuroscience, 18, 794–799. [DOI] [PubMed] [Google Scholar]
- Hornedo‐Ortega, R. , Álvarez‐Fernández, M. A. , Cerezo, A. B. , Richard, T. , Troncoso, A. M. , & Garcia‐Parrilla, M. C. (2016). Protocatechuic acid: Inhibition of fibril formation, destabilization of preformed fibrils of amyloid‐β and α‐synuclein, and neuroprotection. Journal of Agricultural and Food Chemistry, 64, 7722–7732. [DOI] [PubMed] [Google Scholar]
- Howes, M.‐J. R. (2013). Alkaloids and drug discovery for neurodegenerative diseases In Ramawat K. G., & Mérillon J.‐M. (Eds.), Natural products. Phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes (pp. 1331–1365). Berlin Heidelberg: Springer‐Verlag. [Google Scholar]
- Howes, M.‐J. R. (2018). Phytochemicals as anti‐inflammatory nutraceuticals and phytopharmaceuticals In Chatterjee S., Jungraithmayr W., & Bagchi D. (Eds.), Immunity and inflammation in health and disease. Emerging roles of nutraceuticals and functional foods in immune support (pp. 363–388). London, UK: Academic Press (Elsevier). [Google Scholar]
- Howes, M.‐J. R. , Fang, R. , & Houghton, P. J. (2017). Effect of Chinese herbal medicine on Alzheimer's disease In Zeng B.‐Y., & Zhao K. (Eds.), International review of neurobiology. Neurobiology of Chinese herb medicine, vol. 135 (pp. 29–56). London, UK: Academic Press (Elsevier). [DOI] [PubMed] [Google Scholar]
- Howes, M.‐J. R. , & Houghton, P. J. (2009). Traditional medicine for memory enhancement In Ramawat K. G. (Ed.), Herbal drugs: Ethnomedicine to modern medicine (pp. 239–291). Berlin Heidelberg: Springer‐Verlag. [Google Scholar]
- Howes, M.‐J. R. , & Houghton, P. J. (2012). Ethnobotanical treatment strategies against Alzheimer's disease. Current Alzheimer Research, 9, 67–85. [DOI] [PubMed] [Google Scholar]
- Howes, M.‐J. R. , & Perry, E. (2011). The role of phytochemicals in the treatment and prevention of dementia. Drugs & Aging, 28, 439–468. [DOI] [PubMed] [Google Scholar]
- Howes, M.‐J. R. , & Simmonds, M. S. J. (2014). The role of phytochemicals as micronutrients in health and disease. Current Opinion in Clinical Nutrition and Metabolic Care, 17, 558–566. [DOI] [PubMed] [Google Scholar]
- Hwang, S. L. , & Yen, G. C. (2008). Neuroprotective effects of the citrus flavanones against H2O2‐induced cytotoxicity in PC12 cells. Journal of Agricultural and Food Chemistry, 56, 859–864. [DOI] [PubMed] [Google Scholar]
- Ibrahim, A. F. L. , Zickri, M. B. , Aal, L. A. , Heikal, O. , & Osama, E. (2016). The effect of thymoquinone, α7 receptor agonist and α7 receptor allosteric modulator on the cerebral cortex in experimentally induced Alzheimer's disease in relation to MSCs activation. International Journal of Stem Cells, 9, 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida, K. , Yamamoto, M. , Misawa, K. , Nishimura, H. , Misawa, K. , Ota, N. , & Shimotoyodome, A. (2019). Coffee polyphenols prevent cognitive dysfunction and suppress amyloid β plaques in APP/PS2 transgenic mouse. Neuroscience Research. in press [DOI] [PubMed] [Google Scholar]
- Jiang, C.‐Y. , Wang, C. , Xu, N.‐X. , Fujita, T. , Murata, Y. , & Kumamoto, E. (2016). 1,8‐ and 1,4‐Cineole enhance spontaneous excitatory transmission by activating different types of transient receptor potential channels in the rat spinal substantia gelatinosa. Journal of Neurochemistry, 136, 764–777. [DOI] [PubMed] [Google Scholar]
- Jimenez‐Garcia, S. N. , Guevara‐Gonzalez, R. G. , Miranda‐Lopez, R. , Feregrino‐Perez, A. A. , Torres‐Pacheco, I. , & Vazquez‐Cruz, M. A. (2013). Functional properties and quality characteristics of bioactive compounds in berries: Biochemistry, biotechnology, and genomics. Food Research International, 54, 1195–1207. [Google Scholar]
- Kato, M. , Ochiai, R. , Kozuma, K. , Sato, H. , & Katsuragi, Y. (2018). Effect of chlorogenic acid intake on cognitive function in the elderly: A pilot study. Evidence‐Based Complementary and Alternative Medicine, 2018, 8608497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur, N. , Chugh, V. , & Gupta, A. K. (2014). Essential fatty acids as functional components of foods—A review. Journal of Food Science and Technology, 51, 2289–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean, R. J. , Lamport, D. J. , Dodd, G. F. , Freeman, J. E. , Williams, C. M. , Ellis, J. A. , … Spencer, J. P. E. (2015). Chronic consumption of flavanone‐rich orange juice is associated with cognitive benefits: An 8‐wk, randomized, double‐blind, placebo‐controlled trial in healthy older adults. American Journal of Clinical Nutrition, 101, 506–514. [DOI] [PubMed] [Google Scholar]
- Kelley, D. S. , Adkins, Y. , & Laugero, K. D. (2018). A review of the health benefits of cherries. Nutrients, 10, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, D. , Okello, E. , Chazot, P. , Howes, M.‐J. , Ohiomokhare, S. , Jackson, P. , … Wightman, E. (2018). Volatile terpenes and brain function: Investigation of the cognitive and mood effects of Mentha × piperita L. essential oil with in vitro properties relevant to central nervous system function. Nutrients, 10, 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. Y. , Seo, H. J. , Min, S. S. , Park, M. , & Seol, G. H. (2014). The effect of 1,8‐cineole inhalation on preoperative anxiety. A randomized clinical trial. Evidence‐Based Complementary and Alternative Medicine, 2014, 820126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongkeaw, C. , Dilokthornsakul, P. , Thanarangsarit, P. , Limpeanchob, N. , & Norman, S. C. (2014). Meta‐analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. Journal of Ethnopharmacology, 151, 528–535. [DOI] [PubMed] [Google Scholar]
- Lai, P. M. , Collaku, A. , & Reed, K. (2017). Efficacy and safety of topical diclofenac/menthol gel for ankle sprain. A randomized, double‐blind, placebo‐ and active‐controlled trial. The Journal of International Medical Research, 45, 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport, D. J. , Pal, D. , Macready, A. L. , Barbosa‐Boucas, S. , Fletcher, J. M. , Williams, C. M. , & Butler, L. T. (2016). The effects of flavanone‐rich citrus juice on cognitive function and cerebral blood flow: An acute, randomised, placebo‐controlled cross‐over trial in healthy, young adults. British Journal of Nutrition, 116, 2160–2168. [DOI] [PubMed] [Google Scholar]
- Lamport, D. J. , Pal, D. , Moutsiana, C. , Field, D. T. , Williams, C. M. , Spencer, J. P. E. , & Butler, L. T. (2015). The effect of flavanol‐rich cocoa on cerebral perfusion in healthy older adults during conscious resting state: A placebo controlled, crossover, acute trial. Psychopharmacology, 232, 3227–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, S. C. , & Orsini, N. (2018). Coffee consumption and risk of dementia and Alzheimer's disease: A dose–response meta‐analysis of prospective studies. Nutrients, 10, 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Torosyan, N. , & Silverman, D. H. (2017). Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double‐blinded placebo controlled pilot study. Experimental Gerontology, 87, 121–128. [DOI] [PubMed] [Google Scholar]
- Leone, S. , Recinella, L. , Chiavaroli, A. , Orlando, G. , Ferrante, C. , Leporini, L. , … Menghini, L. (2018). Phytotherapic use of the Crocus sativus L. (Saffron) and its potential applications: A brief overview. Phytotherapy Research, 32, 2364–2375. [DOI] [PubMed] [Google Scholar]
- Lila, M. A. , Burton‐Freeman, B. , Grace, M. , & Kalt, W. (2016). Unravelling anthocyanin bioavailability for human health. Annual Review of Food Science and Technology, 7, 375–393. [DOI] [PubMed] [Google Scholar]
- Lim, D. W. , Son, H. J. , Um, M. Y. , Kim, I.‐H. , Han, D. , Cho, S. , & Lee, C.‐H. (2016). Enhanced cognitive effects of demethoxycurcumin, a natural derivative of curcumin on scopolamine‐induced memory impairment in mice. Molecules, 21, 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardaki, Z. I. , Lamari, F. N. , & Margarity, M. (2017). Saffron (Crocus sativus L.) tea intake prevents learning/memory defects and neurobiochemical alterations induced by aflatoxin B1 exposure in adult mice. Neurochemical Research, 42, 2743–2754. [DOI] [PubMed] [Google Scholar]
- Liu, Q.‐P. , Wu, Y.‐F. , Cheng, H.‐Y. , Xia, T. , Ding, H. , Wang, H. , … Xu, Y. (2016). Habitual coffee consumption and risk of cognitive decline/dementia: A systematic review and meta‐analysis of prospective cohort studies. Nutrition, 32, 628–636. [DOI] [PubMed] [Google Scholar]
- Maclin, J. M. A. , Wang, T. , & Xiao, S. (2019). Biomarkers for the diagnosis of Alzheimer's disease, dementia Lewy body, frontotemporal dementia and vascular dementia. General Psychiatry, 32, e100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massee, L. A. , Ried, K. , Pase, M. , Travica, N. , Yoganathan, J. , Scholey, A. , … Pipingas, A. (2015). The acute and sub‐chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: A randomized controlled trial. Frontiers in Pharmacology, 6, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti, G. , & Di Giacomo, S. (2016). Curcumin and resveratrol in the management of cognitive disorders: What is the clinical evidence? Molecules, 21, 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara, R. K. , Kalt, W. , Shidler, M. D. , McDonald, J. , Summer, S. S. , Stein, A. L. , … Krikorian, R. (2018). Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiology of Aging, 64, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci, P. , Tinarelli, C. , Schulz, R.J. , & Polidori, M.C. (2014). Nutraceuticals in cognitive impairment and Alzheimer's disease. Frontiers in Pharmacology, 5, 00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlig, K. , Skoog, I. , Guo, X. , Schütze, M. , Gustafson, D. , Waern, M. , … Lissner, L. (2008). Alcoholic beverages and incidence of dementia: 34‐year follow‐up of the prospective population study of women in Göteborg. American Journal of Epidemiology, 167, 684–691. [DOI] [PubMed] [Google Scholar]
- Miller, M. G. , Hamilton, D. A. , Joseph, J. A. , & Shukitt‐Hale, B. (2018). Dietary blueberry improves cognition among older adults in a randomized, double‐blind, placebo‐controlled trial. European Journal of Nutrition, 57, 1169–1180. [DOI] [PubMed] [Google Scholar]
- Moshiri, M. , Vahabzadeh, M. , & Hosseinzadeh, H. (2015). Clinical applications of saffron (Crocus sativus) and its constituents: A review. Drug Research, 65, 287–295. [DOI] [PubMed] [Google Scholar]
- Nakajima, A. , Aoyama, Y. , Nguyen, T. T. L. , Shin, E. J. , Kim, H. C. , Yamada, S. , … Ohizumi, Y. (2013). Nobiletin, a citrus flavonoid, ameliorates cognitive impairment, oxidative burden, and hyperphosphorylation of tau in senescence‐accelerated mouse. Behavioural Brain Research, 250, 351–360. [DOI] [PubMed] [Google Scholar]
- Nakajima, A. , Aoyama, Y. , Shin, E. J. , Nam, Y. , Kim, H. C. , Nagai, T. , … Yamada, K. (2015). Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer's disease (3XTg‐AD). Behavioural Brain Research, 289, 69–77. [DOI] [PubMed] [Google Scholar]
- Nelson, L. , & Tabet, N. (2015). Slowing the progression of Alzheimer's disease; what works? Ageing Research Reviews, 23, 193–209. [DOI] [PubMed] [Google Scholar]
- Nematolahi, P. , Mehrabani, M. , Karami‐Mohajeri, S. , & Dabaghzadeh, F. (2018). Effects of Rosmarinus officinalis L. on memory performance, anxiety, depression, and sleep quality in university students. A randomized clinical trial. Complementary Therapies in Clinical Practice, 30, 4–28. [DOI] [PubMed] [Google Scholar]
- Okello, E. J. , & Howes, M.‐J. R. (2018). Essential oils and aromas that affect mood and cognition In Murphy P. N. (Ed.), The Routledge international handbook of psychobiology (pp. 195–208). London, UK: Routledge, Taylor & Francis Group, UK. [Google Scholar]
- Okuyama, S. , Minami, S. , Shimada, N. , Makihata, N. , Nakajima, M. , & Furukawa, Y. (2013). Anti‐inflammatory and neuroprotective effects of auraptene, a citrus coumarin, following cerebral global ischemia in mice. European Journal of Pharmacology, 699, 118–123. [DOI] [PubMed] [Google Scholar]
- Olsson, B. , Lautner, R. , Andreasson, U. , Öhrfelt, A. , Portelius, E. , Bjerke, M. , … Zetterberg, H. (2016). CSF and blood biomarkers for the diagnosis of Alzheimer's disease: A systematic review and meta‐analysis. Lancet Neurology, 15, 673–684. [DOI] [PubMed] [Google Scholar]
- Pacheco, S. M. , Soares, M. S. P. , Gutierres, J. M. , Gerzson, M. F. B. , Carvalho, F. B. , Azambuja, J. H. , … Spanevello, R. M. (2018). Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer's type. The Journal of Nutritional Biochemistry, 56, 193–204. [DOI] [PubMed] [Google Scholar]
- Pahwa, P. , & Goel, R. K. (2016). Asparagus adscendens root extract enhances cognition and protects against scopolamine induced amnesia. An in‐silico and in‐vivo studies. Chemico‐Biological Interactions, 260, 208–218. [DOI] [PubMed] [Google Scholar]
- Perry, E. K. , & Howes, M.‐J. R. (2011). Medicinal plants and dementia therapy: Herbal hopes for brain ageing? CNS Neuroscience & Therapeutics, 17, 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, N. S. L. , Menzies, R. , Hodgson, F. , Wedgewood, P. , Howes, M.‐J. R. , Brooker, H. J. , … Perry, E. K. (2018). A randomised double‐blind placebo‐controlled pilot trial of a combined extract of rosemary, sage and melissa, traditional herbal medicines, on the enhancement of memory in normal healthy subjects, including influence of age. Phytomedicine, 39, 42–48. [DOI] [PubMed] [Google Scholar]
- Phillips, C. (2017). Lifestyle modulators of neuroplasticity: How physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plasticity, 2017, 3589271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipingas, A. , Silberstein, R. B. , Vitetta, L. , van Rooy, C. , Harris, E. V. , Young, J. M. , … Nastasi, J. (2008). Improved cognitive performance after dietary supplementation with a Pinus radiata bark extract formulation. Phytotherapy Research, 22, 1168–1174. [DOI] [PubMed] [Google Scholar]
- Pitsikas, N. (2015). The Effect of Crocus sativus L. and its Constituents on Memory: Basic Studies and Clinical Applications. Evidence‐Based Complementary and Alternative Medicine, 2015, 926284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porres‐Martínez, M. , González‐Burgos, E. , Carretero, M. E. , & Gómez‐Serranillos, M. P. (2016). In vitro neuroprotective potential of the monoterpenes α‐pinene and 1,8‐cineole against H2O2‐induced oxidative stress in PC12 cells. Journal of Biosciences, 71, 191–199. [DOI] [PubMed] [Google Scholar]
- Poulose, S. M. , Miller, M. G. , Scott, T. , & Shukitt‐Hale, B. (2017). Nutritional factors affecting adult neurogenesis and cognitive function. Advances in Nutrition, 8, 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin, L. A. , Smart, C. M. , & Amariglio, R. E. (2017). Subjective cognitive decline in preclinical Alzheimer's disease. Annual Review of Clinical Psychology, 13, 369–396. [DOI] [PubMed] [Google Scholar]
- Rafieipour, F. , Hadipour, E. , Emami, S. A. , Asili, J. , & Tayarani‐Najaran, Z. (2019). Safranal protects against beta‐amyloid peptide‐induced cell toxicity in PC12 cells via MAPK and PI3 K pathways. Metabolic Brain Disease, 34, 165–172. [DOI] [PubMed] [Google Scholar]
- Rainey‐Smith, S. R. , Brown, B. M. , Sohrabi, H. R. , Shah, T. , Goozee, K. G. , Gupta, V. B. , & Martins, R. N. (2016). Curcumin and cognition: A randomised, placebo‐controlled, double‐blind study of community‐dwelling older adults. British Journal of Nutrition, 115, 2106–2113. [DOI] [PubMed] [Google Scholar]
- Rakesh, G. , Szabo, S. T. , Alexopoulos, G. S. , & Zannas, A. S. (2017). Strategies for dementia prevention: Latest evidence and implications. Therapeutic Advances in Chronic Disease, 8, 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva, A. K. , & Chopra, K. (2015). Naringin mitigate okadaic acid‐induced cognitive impairment in an experimental paradigm of Alzheimer's disease. Journal of Functional Foods, 19, 110–125. [Google Scholar]
- Saitou, K. , Ochiai, R. , Kozuma, K. , Sato, H. , Koikeda, T. , Osaki, N. , & Katsuragi, Y. (2018). Effect of chlorogenic acids on cognitive function: A randomized, double‐blind, placebo‐controlled trial. Nutrients, 10, 1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian, S. , Farkhondeh, T. , & Samini, F. (2018). A review on possible therapeutic effect of Nigella sativa and thymoquinone in neurodegenerative diseases. CNS & Neurological Disorders Drug Targets, 17, 412–420. [DOI] [PubMed] [Google Scholar]
- Sanei, M. , & Saberi‐Demneh, A. (2019). Effect of curcumin on memory impairment: A systematic review. Phytomedicine, 52, 98–106. [DOI] [PubMed] [Google Scholar]
- Sarker, M. R. , & Franks, S. F. (2018). Efficacy of curcumin for age‐associated cognitive decline: A narrative review of preclinical and clinical studies. GeroScience, 40, 73–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto, A. , Okuyama, S. , Nakajima, M. , & Furukawa, Y. (2019). Citrus flavonoid 3,5,6,7,8,3′,4′‐heptamethoxyflavone induces BDNF via cAMP/ERK/CREB signaling and reduces phosphodiesterase activity in C6 cells. Pharmacological Reports, 71, 653–658. [DOI] [PubMed] [Google Scholar]
- Scholey, A. , & Owen, L. (2013). Effects of chocolate on cognitive function and mood: A systematic review. Nutrition Reviews, 71, 665–681. [DOI] [PubMed] [Google Scholar]
- Selma, M. V. , Espin, J. C. , & Tomas‐Barberan, F. A. (2009). Interaction between phenolics and gut microbiota: Role in human health. Journal of Agricultural and Food Chemistry, 57, 6485–6501. [DOI] [PubMed] [Google Scholar]
- Seo, E.‐J. , Fischer, N. , & Efferth, T. (2018). Phytochemicals as inhibitors of NF‐κB for treatment of Alzheimer's disease. Pharmacological Research, 129, 262–273. [DOI] [PubMed] [Google Scholar]
- Shabani, S. , & Mirshekar, M. A. (2018). Diosmin is neuroprotective in a rat model of scopolamine‐induced cognitive impairment. Biomedicine & Pharmacotherapy, 108, 1376–1383. [DOI] [PubMed] [Google Scholar]
- Shekarchi, M. , Hajimehdipoor, H. , Saeidnia, S. , Gohari, A. R. , & Hamedani, M. P. (2012). Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacognosy Magazine, 8, 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, J. , Xu, L. , Qu, C. , Sun, H. , & Zhang, J. (2018). Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR‐134 signalling pathway regulates CREB/BDNF expression in hippocampus in vivo and in vitro . Behavioural Brain Research, 349, 1–7. [DOI] [PubMed] [Google Scholar]
- Sheng, K. , Shui, S. , Yan, L. , Yu, J. , Hao, G. , Qu, H. , … Zheng, L. (2018). The beneficial effects of dietary grape supplementation on improving cognitive deficits in APP/PS1 double transgenic mice. Journal of Functional Foods, 49, 224–234. [Google Scholar]
- Shoba, G. , Joy, D. , Joseph, T. , Majeed, M. , Rajendran, R. , & Srinivas, P. S. (1998). Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Medica, 64, 353–356. [DOI] [PubMed] [Google Scholar]
- Smeriglio, A. , Barreca, D. , Bellocco, E. , & Trombetta, D. (2017). Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. British Journal of Pharmacology, 174, 1244–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov, A. N. , Pavlova, M. A. , Klosterhalfen, S. , & Enck, P. (2013). Chocolate and the brain: Neurobiological impact of cocoa flavanols on cognition and behavior. Neuroscience and Biobehavioral Reviews, 37, 2445–2453. [DOI] [PubMed] [Google Scholar]
- Solfrizzi, V. , Agosti, P. , Lozupone, M. , Custodero, C. , Schilardi, A. , Valiani, V. , … Panza, F. (2018). Nutritional interventions and cognitive‐related outcomes in patients with late‐life cognitive disorders: A systematic review. Neuroscience and Biobehavioral Reviews, 95, 480–498. [DOI] [PubMed] [Google Scholar]
- SoukhakLari, R. , Moezi, L. , Pirsalami, F. , Ashjazadeh, N. , & Moosavi, M. (2018). Curcumin ameliorates scopolamine‐induced mice memory retrieval deficit and restores hippocampal p‐Akt and p‐GSK‐3β. European Journal of Pharmacology, 841, 28–32. [DOI] [PubMed] [Google Scholar]
- Steiner, G. Z. , Bensoussan, A. , Liu, J. , Hohenberg, M. I. , & Chang, D. H. (2018). Study protocol for a randomised, double‐blind, placebo‐controlled 12‐week pilot phase II trial of Sailuotong (SLT) for cognitive function in older adults with mild cognitive impairment. Trials, 19, 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subash, S. , Braidy, N. , Essa, M. M. , Zayana, A.‐B. , Ragini, V. , Al‐Adawi, S. , … Guillemin, G. J. (2015). Long‐term (15mo) dietary supplementation with pomegranates from Oman attenuates cognitive and behavioral deficits in a transgenic mice model of Alzheimer's disease. Nutrition, 31, 223–229. [DOI] [PubMed] [Google Scholar]
- Thummayot, S. , Tocharus, C. , Suksamrarn, A. , & Tocharus, J. (2016). Neuroprotective effects of cyanidin against Aβ‐induced oxidative and ER stress in SK‐N‐SH cells. Neurochemistry International, 101, 15–21. [DOI] [PubMed] [Google Scholar]
- Tse, K. H. , & Herrup, K. (2017). Re‐imagining Alzheimer's disease—The diminishing importance of amyloid and a glimpse of what lies ahead. Journal of Neurochemistry, 143, 432–444. [DOI] [PubMed] [Google Scholar]
- Tsolaki, M. , Karathanasi, E. , Lazarou, I. , Dovas, K. , Verykouki, E. , Karacostas, A. , … Sinakos, Z. (2016). Efficacy and safety of Crocus sativus L. in patients with mild cognitive impairment: One year single‐blind randomized, with parallel groups, clinical trial. Journal of Alzheimer's Disease, 54, 129–133. [DOI] [PubMed] [Google Scholar]
- Wake, G. , Court, J. , Pickering, A. , Lewis, R. , Wilkins, R. , & Perry, E. (2000). CNS acetylcholine receptor activity in European medicinal plants traditionally used to improve failing memory. Journal of Ethnopharmacology, 69, 105–114. [DOI] [PubMed] [Google Scholar]
- Wang, D. M. , Li, S. Q. , Zhu, X. Y. , Wang, Y. , Wu, W. L. , & Zhang, X. J. (2013). Protective effects of hesperidin against amyloid‐β (Aβ) induced neurotoxicity through the voltage dependent anion channel 1 (VDAC1)‐mediated mitochondrial apoptotic pathway in PC12 cells. Neurochemical Research, 38, 1034–1044. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Zhang, D. , Hu, J. , Jia, Q. , Xu, W. , Su, D. , … Xiao, J. (2017). A clinical and mechanistic study of topical borneol‐induced analgesia. EMBO Molecular Medicine, 9, 802–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengreen, H. , Munger, R. G. , Cutler, A. , Quach, A. , Bowles, A. , Corcoran, C. , … Welsh‐Bohmer, K. A. (2013). Prospective study of dietary approaches to stop hypertension‐ and Mediterranean‐style dietary patterns and age‐related cognitive change: The Cache County Study on Memory. Health and Aging. American Journal of Clinical Nutrition, 98, 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte, A. , Cheng, N. , Fromentin, E. , & Williams, C. (2018). A randomized, double‐blinded, placebo‐controlled study to compare the safety and efficacy of low dose enhanced wild blueberry powder and wild blueberry extract (ThinkBlue™) in maintenance of episodic and working memory in older adults. Nutrients, 10, 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, P. , Sorribas, A. , & Howes, M.‐J. R. (2011). Natural products as a source of Alzheimer's drug leads. Natural Product Reports, 28, 48–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, A. N. , Brenner, M. C. , Punessen, N. , Snodgrass, M. , Byars, C. , Arora, Y. , & Linseman, D. A. (2017). Comparison of the neuroprotective and anti‐inflammatory effects of the anthocyanin metabolites, protocatechuic acid and 4‐hydroxybenzoic acid. Oxidative Medicine and Cellular Longevity, 2017, 6297080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte, A. V. , Kerti, L. , Hermannstädter, H. M. , Fiebach, J. B. , Schreiber, S. J. , Schuchardt, J. P. , & Flöel, A. (2013). Long‐chain omega‐3 fatty acids improve brain function and structure in older adults. Cerebral Cortex, 24, 3059–3068. [DOI] [PubMed] [Google Scholar]
- Wongsa, P. , Khampa, N. , Horadee, S. , Chaiwarith, J. , & Rattanapanone, N. (2019). Quality and bioactive compounds of blends of Arabica and Robusta spray‐dried coffee. Food Chemistry, 283, 579–587. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Sun, D. , & He, Y. (2017). Coffee intake and the incident risk of cognitive disorders: A dose–response meta‐analysis of nine prospective cohort studies. Clinical Nutrition, 36, 730–736. [DOI] [PubMed] [Google Scholar]
- Yu, M. , Chen, X. , Liu, J. , Ma, Q. , Zhuo, Z. , Chen, H. , … Hou, S.‐T. (2019). Gallic acid disruption of Aβ1–42 aggregation rescues cognitive decline of APP/PS1 double transgenic mouse. Neurobiology of Disease, 124, 67–80. [DOI] [PubMed] [Google Scholar]
- Zaplatic, E. , Bule, M. , Shah, S. Z. A. , Uddin, M. S. , & Niaz, K. (2019). Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer's disease. Life Sciences, 224, 109–119. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Wang, Y. , Dong, X. , & Liu, J. (2018). Crocetin attenuates inflammation and amyloid‐β accumulation in APPsw transgenic mice. Immunity and Ageing, 15, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, A. , Taylor, A. E. , Karhunen, V. , Zhan, Y. , Rovio, S. P. , Lahti, J. , … Hyppönen, E. (2018). Habitual coffee consumption and cognitive function: A Mendelian randomization meta‐analysis in up to 415,530 participants. Scientific Reports, 8, 7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, C. W. , Grossman, H. , Neugroschl, J. , Parker, S. , Burden, A. , Luo, X. , & Sano, M. (2018). A randomized, double‐blind, placebo‐controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer's disease: A pilot study. Alzheimer's and Dementia: Translational Research and Clinical Interventions, 4, 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L.‐N. , Mei, X. , Zhang, Z.‐G. , Xie, Y.‐P. , & Lang, F. (2019). Curcumin intervention for cognitive function in different types of people: A systematic review and meta‐analysis. Phytotherapy Research, 33, 524–533. [DOI] [PubMed] [Google Scholar]


