Figure 1: Developmental shift from excitation to inhibition by GABA-A receptors in rat medial prefrontal cortex slices is delayed by perinatal cannabinoid exposure.
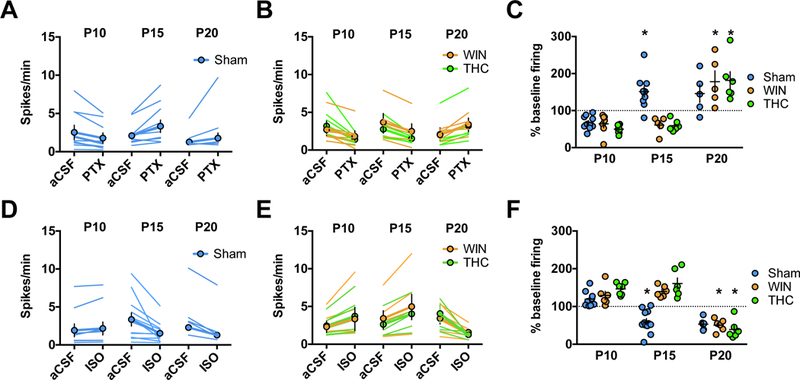
Action potentials were recorded in cell-attached (I=0) layer 5 pyramidal neurons in standard aCSF. After 10 min of baseline recording, picrotoxin (20 μM; GABA-A receptor antagonist, PTX) or isoguvacine (7 μM; GABA-A receptor agonist, ISO) was bath-applied. Spiking activity was calculated as an average of spikes per minute (10 min baseline) compared to the last 10 min of drug application. A-C: GABA-A receptor antagonism is inhibitory in immature P09-P10 mPFC networks in the progeny of Sham-, WIN-, or THC-treated dams but excitatory at P15 in Sham-exposed offspring and P21 in WIN- or THC-exposed offspring. PTX decreased spike frequency in slices obtained from P09–10 rats (Sham: N=8 cells/5 rats, WIN: N=6 cells/4 rats, THC N=7 cells/5 rats). In contrast, PTX increased spike frequency in slices obtained from Sham-treated P15–16 rats (N=9 cells/6 rats) while continuing to decrease spike frequency in slices obtained from WIN- or THC-exposed rats (WIN: N=5 cells/4 rats, THC: N=5 cells/4 rats). At P20–21, PTX application increased spike frequency in slices obtained from either Sham-, WIN-, or THC-exposed rats (Sham: N=5 cells/4 rats, WIN: N=5 cells/4 rats, THC: N=4 cells/4 rats). Two-way ANOVA revealed a significant drug/postnatal day interaction (F4,35 = 6.479, P=0.0003; * indicates P<0.05 as compared to respective P10 normalized post-drug firing rate as determined by Tukey’s post-hoc analysis. Error bars indicate SEM. Example traces shown in Supplementary Figure 4. D-F: GABA-A receptor agonism is excitatory in immature P09–10 mPFC networks in the progeny of Sham-, WIN, or THC-treated dams but inhibitory at P15 in Sham-exposed offspring and P21 in WIN- or THC-exposed offspring. ISO increased spike frequency in slices obtained from P09–10 rats (Sham: N=9 cells/rats, WIN: N=6 cells/4 rats, THC: N=7 cells/4 rats). In contrast, ISO application decreased spike frequency in slices obtained from P15–16 pups from Sham-treated dams (N=11 cells/7 rats) while it continued to increase spike frequency in slices obtained from P15–16 pups from WIN- or THC-treated dams (WIN: N=6 cells/rats, THC: N=6 cells/4 rats). At P20–21, ISO application decreased spike frequency in slices obtained from the offspring of all conditions (Sham: N=6 cells/4 rats, WIN: N=6 cells/4 rats, THC: N=6 cells/4 rats). Two-way ANOVA revealed a significant drug/postnatal day interaction (F4,55 = 12.94, P<0.0001; * indicates P<0.05 as compared to respective P10 normalized post-drug firing rate as determined by Tukey’s post-hoc analysis. Error bars indicate SEM. Example traces shown in Supplementary Figure 5–6.
