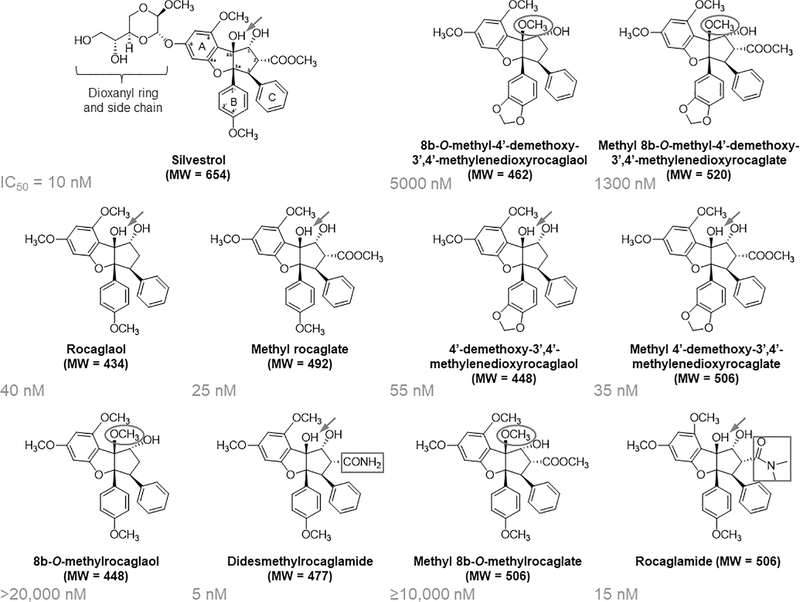
Figure 1. Identification of didesmethylrocaglamide and rocaglamide with potent growth-inhibitory activity comparable to silvestrol.
The structure of each rocaglate is shown along with its IC50 value in STS26T MPNST cells as determined in Table 1. Structure-activity comparison revealed that the dioxanyl (dioxanyloxy) ring is dispensable but may enhance the cytotoxicity of rocaglates. An unmethylated C-8b hydroxyl group (arrow) and the amide functionality (rectangle) of didesmethylrocaglamide and rocaglamide are important for optimum antiproliferative activity, while methylation of the C-8b hydroxyl group (oval) substantially impaired the activity.