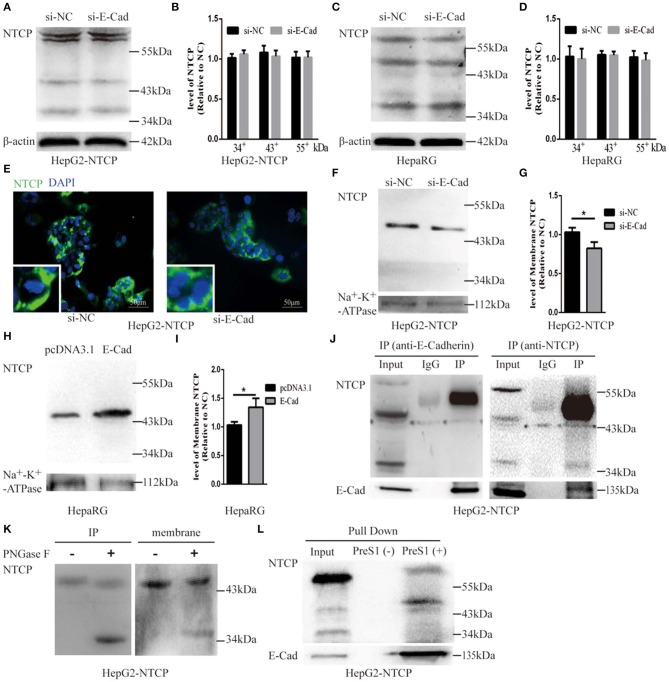
Figure 6.
E-cadherin facilitates NTCP localization to the cell surface through interacting with glycosylated NTCP. Total protein was extracted 3 days post-transfection with siRNA-NC or siRNA-E-cadherin. NTCP expression was assessed by western blot analysis in (A) HepG2-NTCP and (C) HepaRG cells. The samples were derived from the same experiment and gels were processed in parallel. (B,D) The densitometric ratios of every band were normalized to the β-actin and then compared to the controls. There was no significant difference between the groups. (E) NTCP expression was assessed by immunofluorescence 3 days post-transfection with E-cadherin siRNA in HepG2-NTCP cells. (F) Membrane protein was extracted 3 days post-transfection with siRNA-NC (left panel) or siRNA-E-cadherin (right panel) and NTCP expression was assessed by western blot analysis in HepG2-NTCP cells. (G) The densitometric ratios were normalized to the Na+-K+-ATPase and then compared to the controls. (H) Membrane protein was extracted 4 days post-transfection with pcDNA3.1 plasmid or pcDNA3.1-E-cadherin and NTCP expression was assessed by western blot analysis in HepaRG cells. (I) The densitometric ratios were normalized to the Na+-K+-ATPase and then compared to the controls. (J) Co-immunoprecipitation was performed to confirm the interaction between E-cadherin and NTCP. Input: Total protein from cell extract. IgG of rabbit was used as the control group. IP: Total protein from Hep2-NTCP cells was incubated with anti-E-cadherin, or anti-NTCP at 4°C overnight. (K) The precipitate of IP and membrane protein were treated by PNGase F at 37°C for 1 h and detected by western blot. (L) The HepG2-NTCP cell lysates were incubated with pre-S1 to confirm that preS1 was bound to glycosylated NTCP. Representative data is shown from triplicate experiments. *p < 0.05; error bars: standard deviation (SD).