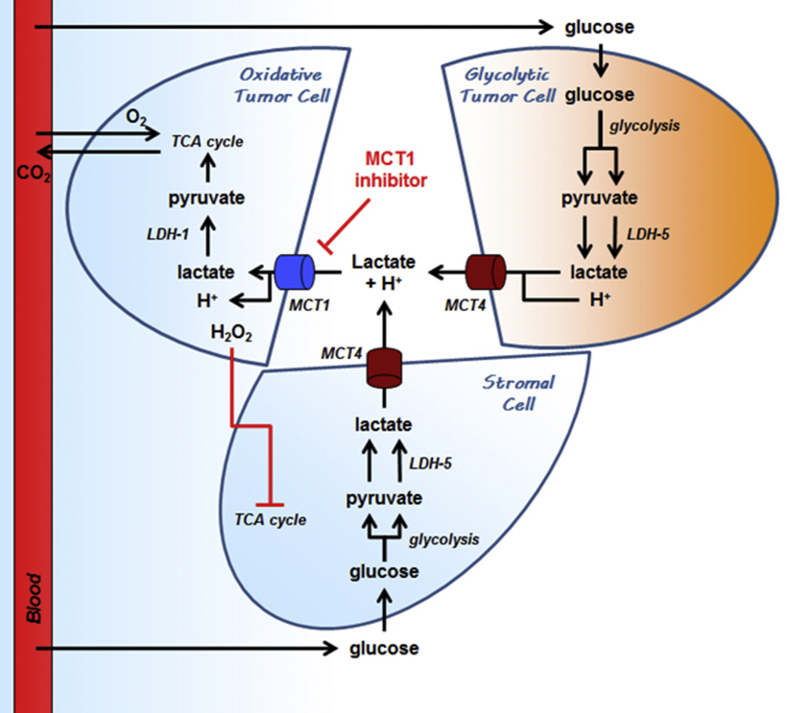
Figure 2.
Metabolic symbiosis and commensalism based on the exchange of lactate in cancer. The cartoon depicts a tumor-feeding blood vessel delivering glucose and oxygen to cancer cells. An oxidative cancer cell is represented close to the blood vessel, a hypoxic cancer cells remotely, and a host cell on the bottom. From a metabolic standpoint, the hypoxic cancer cell has no choice but to perform anaerobic glycolysis to survive, which implies having access to high amounts of glucose. Comparatively, the oxidative cancer cell can use several different metabolic fuels. When nearby glycolytic cells provide lactate (usually a MCT4-dependent process), it uses lactate as an oxidative fuel preferentially to glucose (usually a MCT1-dependent process), which increases glucose availability for the glycolytic cancer cell. The oxidative cancer cell can obtain additional lactate by forcing the host cell to adopt a glycolytic metabolism. When lactate is not available or when MCTs are inhibited, the oxidative cancer cell switches to a glucose-based metabolism, thus depriving other cells from this important resource, which ultimately kills the hypoxic cancer cell. MCT1 and MCT4 inhibitors can, thus, destroy both the metabolic symbiosis and the commensalism based on the exchange of lactate in cancer. For abbreviations, see list. Adapted from reference [219].