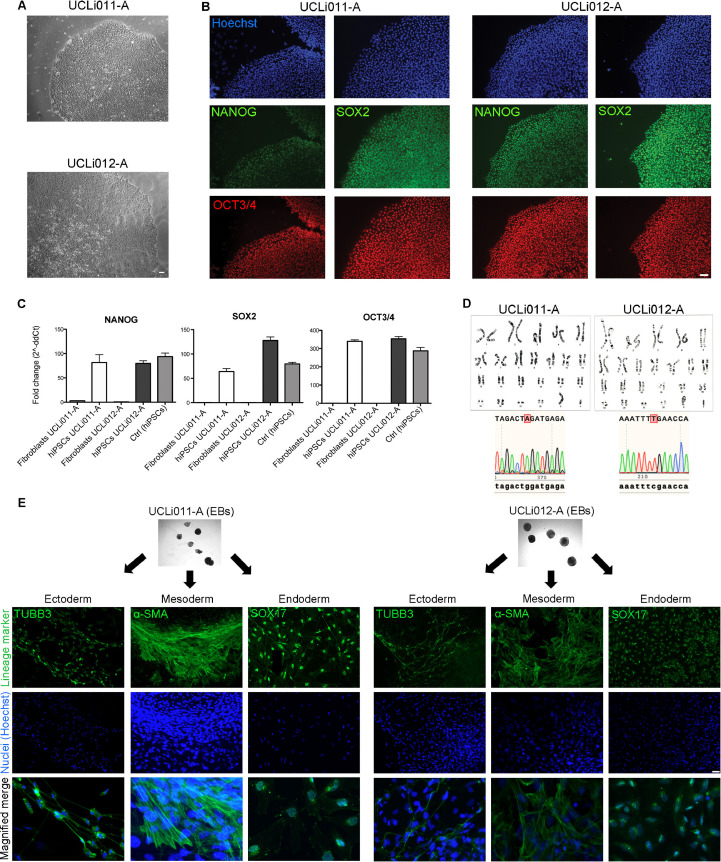
Fig. 1.
(A) Phase contrast images showing colonies of both DMD iPSC lines. Scale bar 100 μm. (B) Immunofluorescence staining showing pluripotency-associated markers (NANOG, SOX2 and OCT3/4) in UCLi011-A and UCLi012-A iPSCs. Hoechst: nuclei. Scale bar: 75 μm. (C) Quantitative real-time PCR analysis showing expression of mRNAs of pluripotency-associated factors (NANOG, SOX2 and OCT3/4) in UCLi011-A and UCLi012-A iPSCs and their absence in parental fibroblasts. (D) Upper images: normal karyotype of UCLi011-A and UCLi012-A iPSCs (46,XY). Lower images: electropherograms confirming presence of pathogenic mutations in exon 68 (c.9851G>A (p.Trp3284X) and 70 c.10141C>T (p.Arg3381X) of the dystrophin gene. (E) Embryoid body formation assay. Upper phase contract images show morphology of UCLi011-A and UCLi012-A embryoid bodies. Lower panels show immunofluorescence staining of the same embryoid bodies with lineage-specific markers: α-smooth muscle actin (mesoderm), βIII-tubulin (ectoderm) and SOX17 (endoderm). Hoechst: nuclei. Scale bar: 75 μm. Bottom images: merged pictures showing magnified areas of each lineage-specific staining.