Figure 1.
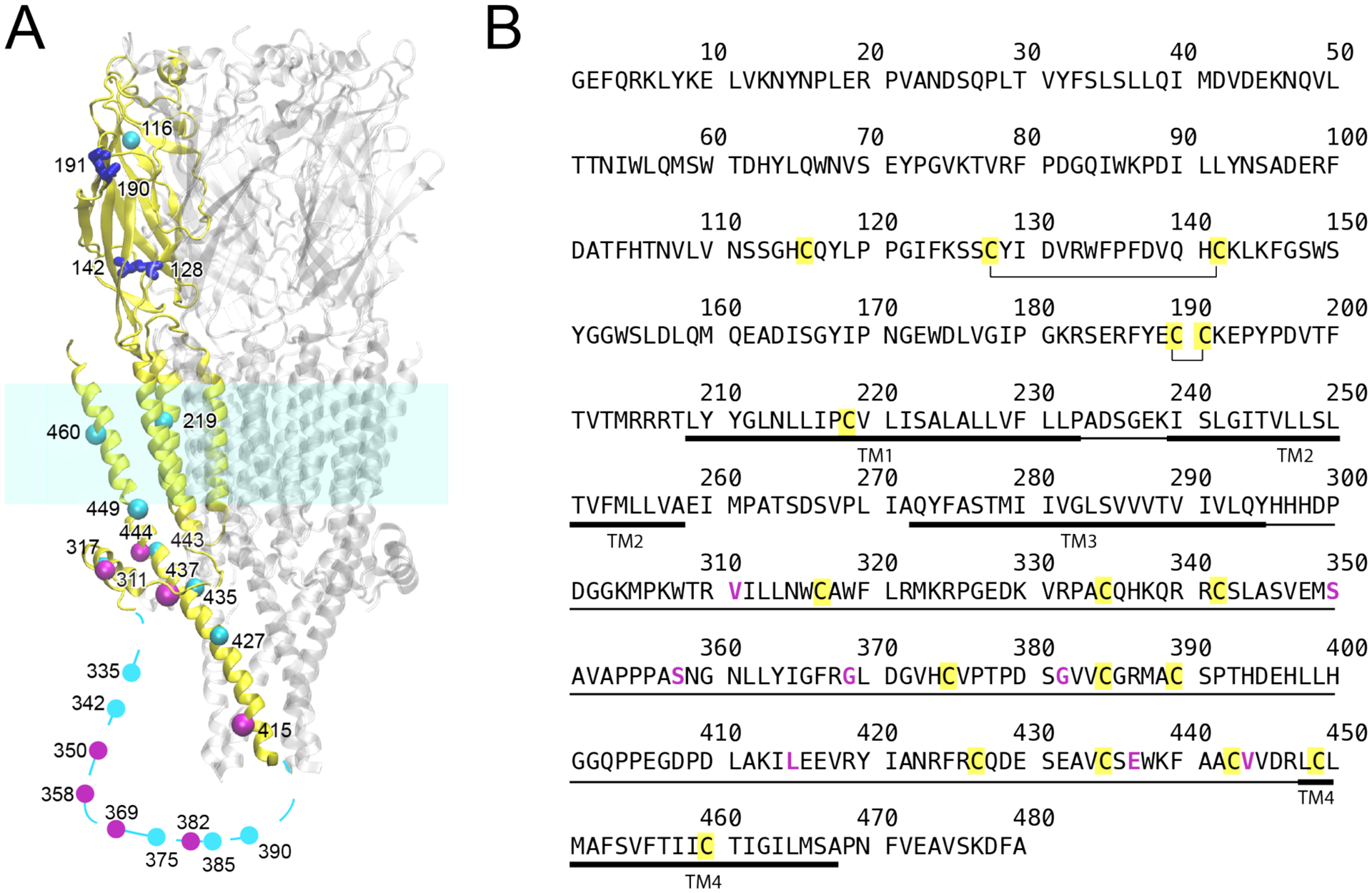
(A) Homology structure of human α7 nAChR showing cysteine positions. For clarity, cysteines are highlighted only in a single subunit (yellow). Native cysteines forming disulfide bonds are in blue; native cysteines mutated to create the Cys-min construct are in cyan; non-native cysteines introduced by mutation are in magenta. Cysteines in the transmembrane domain were mutated to alanine. All other cysteines, except four cysteines in blue, were mutated to serine. The homology model was based on 5HT3AR (PDB ID: 6BE1). The dotted line represents a region in the intracellular domain where no homology structure is available. (B) Sequence of human α7 nAChR. Disulfide bonds are annotated, native cysteines are marked in yellow, and residues mutated to cysteines are magenta. The intracellular domain and the transmembrane domain are marked in thin and thick lines, respectively.
