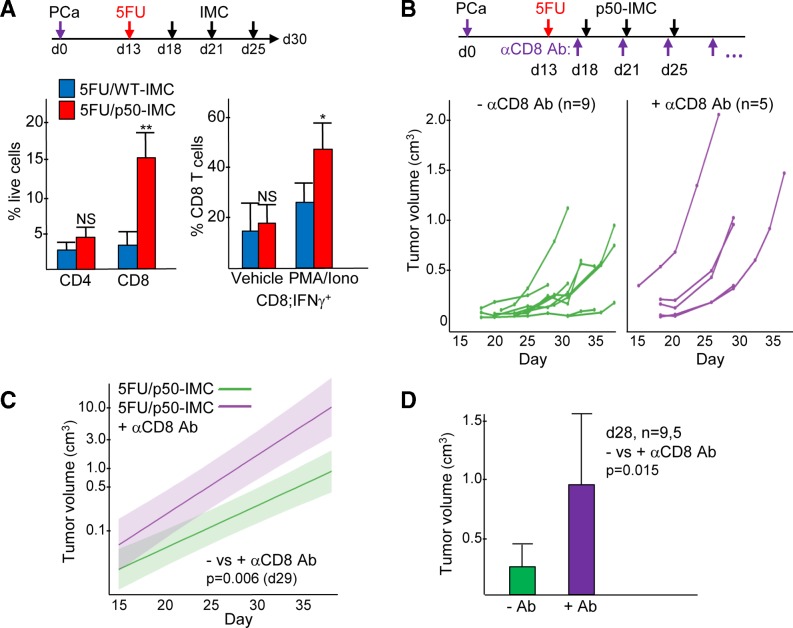
Figure 7.
p50-IMC increases PCa tumor-activated CD8+ T cells numbers, and CD8+ T cells are required for efficacy of 5FU/p50-IMC. (A) Mice inoculated with Hi-Myc PCa on day 0 received 5FU on day 13 and 107 WT-IMC (n=5) or p50-IMC (n=4) on days 18, 21 and 25, followed by analysis of cells obtained from individual tumors on day 30 from a single experiment, as diagrammed. CD3+CD4+ and CD3+CD8+ T cells were enumerated as a percent of viable cells using flow cytometry (left). Single suspensions of tumor cells were placed in culture with DMEM/fetal bovine serum vehicle alone or with PMA/ionomycin for 4 hours, followed by analysis of CD3+CD8+ T cells for intracellular IFNγ (right). (B) Mice inoculated with Hi-Myc PCa on day 0 received 5FU on day 13 and 107 WT-IMC or p50-IMC on days 18, 21 and 25 with or without CD8 antibody treatment two times per day weekly starting on day 17, as diagrammed. Individual tumor volumes as measured every 2–4 days, combined from two experiments, are shown. (C) These data were fit to an exponential model, with semilog plots and comparison of expected mean tumor volumes between the 5FU/p50-IMC and 5FU/p50-IMC plus anti-CD8 antibody (αCD8 Ab) groups on day 29. (D) Tumor volumes as measured on day 18 for these two groups are also compared. 5FU, 5-fluorouracil; DMEM, Dulbecco’s modified Eagle medium; IFNγ, interferon γ; IMC, immature myeloid cell; NS, not significant; p50-IMC, p50-negative immature myeloid cell; PCa, prostate cancer; PMA, phorbol myristate acetate; WT, wild type.