Abstract
The way to a successful vaccine against human cytomegalovirus is hampered by the peculiar biology of this infection. However, some candidate vaccines have been shown to protect seronegative women and transplant recipients, and we should know soon whether they can prevent congenital infection.
The history of cytomegalovirus (CMV) vaccines began in the 1970s when Elek and Stern [1] in England and Plotkin and associates [2] in the United States developed live attenuated strains of the virus and tested them in adult volunteers. Both strains were well tolerated and immunogenic, but only development of work on the United States strain was continued. Kidney transplant recipients who were CMV seronegative and who received a kidney from a seropositive donor are at high risk of CMV disease, but vaccination of recipients with the Towne strain greatly reduced serious symptoms and graft rejection [3]. Early work on a subunit vaccine using glycoprotein B (gB) also seemed promising [4, 5]. However, commercial enthusiasm for a CMV vaccine was limited until 2000, when the Institute of Medicine (now the National Academy of Medicine) issued a report on needed vaccines and placed a CMV vaccine in its highest priority [6]. That report greatly accelerated interest in CMV by the major manufacturers. The development of vaccines against CMV is now considered an important goal [7–10].
Years of work have identified the antigens that are likely to be important for vaccination: the gB surface glycoprotein; the pentamer protein complex, also on the surface of the virus; the gH/gL/g0 trimer; and the pp65 tegument protein. The first 3 generate antibodies with important functions, and the last one is the most important inducer of T-cellular immune responses. The immediate early proteins are also included in some vaccines as generators of T-cell responses.
The gB glycoprotein has had the most study as a vaccine antigen [4]. Antibodies to gB prevent entry of CMV into fibroblasts. A vaccine was developed originally by Chiron (now GlaxoSmithKline), formulated with vaccine gB antigen and MF-59, an oil-in-water adjuvant [5]. That gB-based vaccine was subsequently developed by Sanofi Pasteur. Human trials with the gB/MF-59 vaccine have been conducted in women with the aim of preventing viral acquisition, and in solid organ transplant patients with the aim of preventing posttransplantation CMV disease. The gB vaccine was moderately successful in preventing acquisition of CMV by seronegative women, (although antibodies and protection waned with time), and it also boosted antibodies in seropositive women [11–13]. gB was also strikingly successful in reducing CMV viremia and the need for treatment in recipients of kidney or liver transplants [14]. Thus, gB is the leading antigen for inclusion in a potential CMV vaccine, particularly in its trimeric form, which is more immunogenic [15].
However, in 2005 an important discovery was made, namely, that another antigen on the surface of the virus—which comprised 5 separate proteins, forming a pentamer—generated the majority of neutralizing antibodies in humans, in particular those that prevented entry into epithelial cells [16]. Moreover, studies in pregnant women conducted at the University of Pavia revealed that the immune response that most closely correlated with prevention of transmission of CMV from infected seronegative pregnant women to their fetuses was a rapid increase in antibody to the pentamer [17]. Thus, the pentamer seems to be another promising antigen for a maternal vaccine that provides fetal protection.
T-cell responses may also be needed: functional CD4+ T cells to prevent congenital infection through promotion of neutralizing antibodies and perhaps through stimulation of other antibody functions, and CD8+ T cells to prevent CMV transplant disease. As mentioned above, the pp65 tegument protein seems to be best for induction of the CD8+ T cells, as shown through its incorporation into various vectors with resultant strong CD8+ T-cell responses [18]. However, in the rhesus monkey, fetal infection with rhesus CMV is prevented by immunoglobulin alone [19].
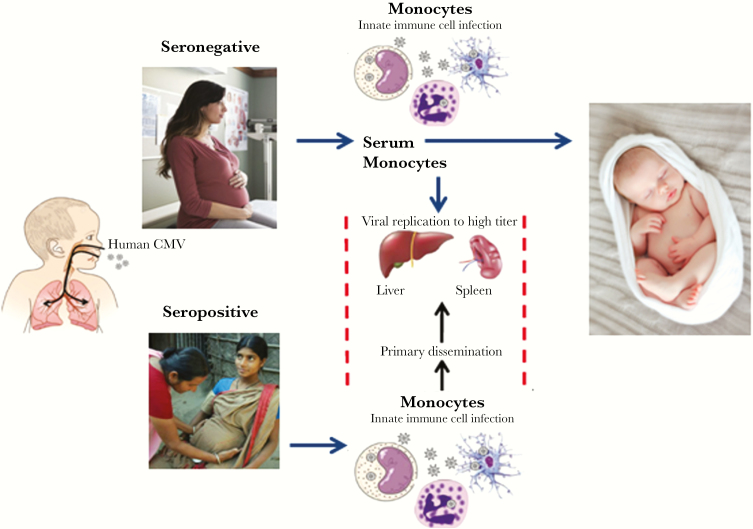
Another issue has recently come to the fore, and that is whether CMV spreads from the nasopharynx through cell-free viremia or cell-associated virus. The evidence suggests that reinfection in seropositive women succeeds through cellular infection in the presence of antibody [20]. On the other hand, the first infection in seronegative women may generate some cell-free viremia that can cross the placenta and infect the fetus, which would explain why fetal transmission is more common in seronegative women [21, 22]. Figure 1 illustrates this concept [23]. Thus, for maximum efficacy, a vaccine that induces both antibodies and cytotoxic T cells may be important, although even a vaccine that only prevents cell-free virus would be valuable for seronegative women.
Figure 1.
Proposed scheme for cytomegalovirus (CMV) infection in pregnancy. Women are often exposed to toddlers excreting CMV. Both seronegative and seropositive women can be exposed, particularly the latter if they live in countries where children are often infected. The CMV infection in both cases rapidly becomes intracellular, although first infections in seronegative women are likely to include cell-free virus in the plasma. In both types of women, CMV spreads to multiple organs. In seronegative women, spread to the placenta cells and on to the fetus is likely, whereas in seropositive women who are repeatedly infected, the placenta usually but not always remains virus free. Thus, seropositivity is a relative protective factor. (Source: Jackson and Sparer [23].)
A major source of controversy is whether immune responses to CMV can protect against infection of pregnant women and passage of virus to their fetuses. Because seropositive women sometimes do transmit CMV to their fetuses, some workers question whether immunization of these women can add to protection. However, the situation is not black and white. A study by Leruez-Ville et al [21] in France concluded that transmission to the fetus from seropositive women occurred at a rate one-fourth of the rate of transmission from seronegative women. Those authors also observed that there was a major socioeconomic difference between the 2 groups, in that seropositive transmitters were more likely to be poor and to be exposed to more children, and thus repeatedly exposed to CMV.
Simonazzi et al [22] in Italy also found that whereas seropositive women could transmit CMV to their fetuses, this occurred at a rate of only 3%, although the transmission rate from seronegative women is thought to be 30%. However, studies in Brazilian women, who are almost all seropositive, showed rates of transmission as high as 6% [24]. In addition, antibodies to gB and the pentamer did not seem to prevent transmission to the fetus from all infected Brazilian women, although only the titer of those antibodies was studied, and not the speed of production [25].
In this writer’s opinion, one must distinguish 2 types of population in order to understand CMV transmission. It seems that induction of antibodies in exposed previously seronegative women can prevent transmission of CMV to their fetuses, although the specificity and function of those antibodies are not completely characterized. Seropositive women are partly protected against transmission to the fetus, but that protection can be overcome if women are exposed to CMV on multiple occasions. Thus, for example, seropositive women in the United States, France, and Italy will have relatively strong protection against transmission to their fetuses, whereas women in Brazil and other poor countries in which exposure to CMV is common and frequent will have less protection against fetal infection. The situation is analogous to that of human immunodeficiency virus, in which protection may be seen after first exposures but the likelihood of infection increases with repeated challenges [26].
It is important to distinguish the various situations in which a CMV vaccine would be useful. The most obvious is to immunize a seronegative woman against primary CMV infection by contact with an infected child or adult. However, as mentioned above, seropositive women may also be reinfected by CMV [24, 27]. A key question is whether the risk to the fetus is as great during reinfection of the mother as in the situation of primary infection. Because seropositive women sometimes do transmit CMV to their fetuses, some workers question whether their immunization of these women can add to protection [28]. In addition, CMV can reactivate in seropositive subjects under conditions of immunosuppression, and pregnancy may be in some sense in that category. However, almost nothing is known about reactivation of CMV as a cause of congenital infection, other than the likelihood that such reactivation does occur during pregnancy and must be accounted as a threat to the fetus.
Separate goals of vaccination against CMV include prevention of primary infection in solid organ transplant recipients and prevention of reactivation or reinfection in hematopoietic stem cell transplant recipients who are seropositive [29, 30]. So far, CMV vaccines have worked better in solid organ transplant recipients to protect them against primary infection. Prevention of reactivation, as in hematogenous stem cell recipients has proven more difficult, probably because that population is more immunosuppressed. Thus, CMV vaccines have multiple indications. Despite the numerous candidate vaccines in trial, there are many unanswered questions about prevention of CMV, including whether infection in seropositive women can be prevented by induction of antibodies or cellular responses, whether T-cell responses are useful to prevent infection in seronegative women, and whether protective responses can be prolonged over the age of childbearing.
CURRENT CANDIDATE CMV VACCINES
Table 1 lists the candidate vaccines known to the author, according to their content, their targets, and the stage of their testing. The companion article [31] gives details about those candidates.
Table 1.
Candidate Cytomegalovirus Vaccines in Development
| Sponsor | Vaccine Type | Study Phase | Target |
|---|---|---|---|
| Merck | Live, replication defective | Phase 2 | Congenital infection |
| Sanofi | gB, pentamer subunit | Phase 2 Preclinical | Congenital infection |
| City of Hope | pp65 subunit, adjuvant | Phase 1 | Infection in transplant recipients |
| City of Hope | MVA presenting pp65, IE1, IE2 | Phase 2 | Infection in transplant recipients |
| Moderna | gB, pentamer mRNA | Phase 2 | Congenital infection |
| GlaxoSmithKline | gB, pentamer subunit, adjuvant | Preclinical | Congenital infection |
| Hookipa | LCMV vector gB, p65 | Phase 1 | Infection in transplant recipients |
| Variations Bio | gB | Phase 1 | Congenital infection |
| Serum Institute of India | Dense bodies | Preclinical | Infection in transplant recipients |
| Queensland Institute | gB, pp65, p50 Polypeptide with TLR-9 adjuvant | Preclinical | Congenital infection |
| Pfizer | Approach not public | Preclinical | Congenital infection |
| Astellas | DNA | Failure | Infection in transplant recipients |
Abbreviations: gB, glycoprotein B; IE1 and IE2, immediate early protein 1 and 2; LCMV, lymphocytic choriomeningitis virus; mRNA, messenger RNA; MVA, modified vaccinia Ankara; TLR-9, Toll-like receptor 9.
HOW TO LICENSE AND USE A CMV VACCINE
There are various ways to demonstrate the efficacy of a CMV vaccine. These include artificial challenge with low-passage virus, cohort studies in pre-pregnant women to prevent fetal infection at birth or to prevent fetal disease, and prevention of disease or infection in solid organ and hematogenous transplant recipients or infection in children in daycare or their mothers.
The Food and Drug Administration in the United States has indicated a preference for a study in which women would be vaccinated before becoming pregnant and then followed up to test their infants for CMV infection [32]. The latter would be easy to determine, because essentially 100% of infected infants will excrete CMV in saliva and urine at birth, detectable by means of polymerase chain reaction or virus isolation. However, such a study would be long and expensive. In addition, large and frequent challenges by exposure of mothers to toddlers excreting CMV might give a false-negative result for protection, so some measure of the size and extent of exposure will be necessary to evaluate vaccine efficacy. Much easier would be to demonstrate protection of nonpregnant women against artificial challenge with a low-passage wild CMV, or prevention of acquisition of CMV by vaccinated women exposed to children in daycare.
Efficacy in solid organ transplant recipients by prior vaccination has already been demonstrated but would need confirmation in larger studies [3, 14]. Once licensed, a CMV vaccine could be given to girls at the age of puberty, women contemplating pregnancy, and even universally to toddlers to reduce their excretion of CMV and resultant contact exposure of their mothers [33].
In summary, there are promising candidate CMV vaccines with partial proof of their efficacy in preventing virus acquisition by seronegative persons and modifying posttransplantation disease. What is lacking is definitive proof of efficacy in preventing congenital infection in infants born to initially seronegative or seropositive women, which we hope will become available in coming years through licensure of a vaccine or vaccines.
Note
Supplement sponsorship. This supplement was sponsored by NIAID and NICHD.
Potential conflicts of interest. The author is a paid consultant to many of the groups developing CV vaccines. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Elek SD, Stern H. Development of a vaccine against mental retardation caused by cytomegalovirus infection in utero. Lancet 1974; 1:1–5. [DOI] [PubMed] [Google Scholar]
- 2. Plotkin SA, Furukawa T, Zygraich N, Huygelen C. Candidate cytomegalovirus strain for human vaccination. Infect Immun 1975; 12:521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Plotkin SA, Smiley ML, Friedman HM, et al. . Towne-vaccine-induced prevention of cytomegalovirus disease after renal transplants. Lancet 1984; 1:528–30. [DOI] [PubMed] [Google Scholar]
- 4. Gonczol E, Ianacone J, Ho WZ, Starr S, Meignier B, Plotkin S. Isolated gA/gB glycoprotein complex of human cytomegalovirus envelope induces humoral and cellular immune-responses in human volunteers. Vaccine 1990; 8:130–6. [DOI] [PubMed] [Google Scholar]
- 5. Pass RF, Duliegè AM, Boppana S, et al. . A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J Infect Dis 1999; 180:970–5. [DOI] [PubMed] [Google Scholar]
- 6. Institute of Medicine Committee to Study Priorities for Vaccine Development. The National Academies Collection: reports funded by National Institutes of Health. In: Stratton KR, Durch JS, Lawrence RS, eds. Vaccines for the 21st century: a tool for decisionmaking. Washington, DC: National Academies Press, 2000. [PubMed] [Google Scholar]
- 7. Griffiths P, Plotkin S, Mocarski E, et al. . Desirability and feasibility of a vaccine against cytomegalovirus. Vaccine 2013; 31(suppl 2):B197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schleiss MR, Berka U, Watson E, et al. . Additive protection against congenital cytomegalovirus conferred by combined glycoprotein B/pp65 vaccination using a lymphocytic choriomeningitis virus vector. Clin Vaccine Immunol 2017; 24. doi: 10.1128/cvi.00300-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Permar SR, Schleiss MR, Plotkin SA. Advancing our understanding of protective maternal immunity as a guide for development of vaccines to reduce congenital cytomegalovirus infections. J Virol 2018; 92. doi: 10.1128/jvi.00030-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Plotkin SA, Boppana SB. Vaccination against the human cytomegalovirus. Vaccine 2018. doi: 10.1016/j.vaccine.2018.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pass RF, Zhang C, Evans A, et al. . Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009; 360:1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sabbaj S, Pass RF, Goepfert PA, Pichon S. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. J Infect Dis 2011; 203:1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernstein DI, Munoz FM, Callahan ST, et al. . Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: a randomized clinical trial. Vaccine 2016; 34:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Griffiths PD, Stanton A, McCarrell E, et al. . Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 2011; 377:1256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cui X, Cao Z, Wang S, et al. . Novel trimeric human cytomegalovirus glycoprotein B elicits a high-titer neutralizing antibody response. Vaccine 2018; 5580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 2005; 102:18153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lilleri D, Gerna G. Maternal immune correlates of protection from human cytomegalovirus transmission to the fetus after primary infection in pregnancy. Rev Med Virol 2017; 27. doi: 10.1002/rmv.1921. [DOI] [PubMed] [Google Scholar]
- 18. Berencsi K, Gyulai Z, Gönczöl E, et al. . A canarypox vector-expressing cytomegalovirus (CMV) phosphoprotein 65 induces long-lasting cytotoxic T cell responses in human CMV-seronegative subjects. J Infect Dis 2001; 183:1171–9. [DOI] [PubMed] [Google Scholar]
- 19. Nelson CS, Huffman T, Jenks JA, et al. . HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc Natl Acad Sci U S A 2018; 115:6267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nguyen CC, Kamil JP. Pathogen at the gates: human cytomegalovirus entry and cell tropism. Viruses 2018; 10. doi: 10.3390/v10120704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leruez-Ville M, Magny JF, Couderc S, et al. . Risk factors for congenital cytomegalovirus infection following primary and nonprimary maternal infection: a prospective neonatal screening study using polymerase chain reaction in saliva. Clin Infect Dis 2017; 65:398–404. [DOI] [PubMed] [Google Scholar]
- 22. Simonazzi G, Curti A, Cervi F, et al. . Perinatal outcomes of non-primary maternal cytomegalovirus infection: a 15-year experience. Fetal Diagn Ther 2018; 43:138–42. [DOI] [PubMed] [Google Scholar]
- 23. Jackson JW, Sparer T. There is always another way! cytomegalovirus' multifaceted dissemination schemes. Viruses 2018; 10. doi: 10.3390/v10070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, et al. . Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis 2009; 49:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vanarsdall AL, Chin AL, Liu J, et al. . HCMV trimer- and pentamer-specific antibodies synergize for virus neutralization but do not correlate with congenital transmission. Proc Natl Acad Sci U S A 2019; 116:3728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petitdemange C, Kasturi SP, Kozlowski PA, et al. . Vaccine induction of antibodies and tissue-resident CD8+ T cells enhances protection against mucosal SHIV-infection in young macaques. JCI Insight 2019; 4. doi: 10.1172/jci.insight.126047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boucoiran I, Mayer BT, Krantz EM, et al. . Nonprimary maternal cytomegalovirus infection after viral shedding in infants. Pediatr Infect Dis J 2018; 37:627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Britt WJ. Congenital human cytomegalovirus infection and the enigma of maternal immunity. J Virol 2017; 91. doi: 10.1128/jvi.02392-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lumbreras C, Manuel O, Len O, ten Berge IJ, Sgarabotto D, Hirsch HH. Cytomegalovirus infection in solid organ transplant recipients. Clin Microbiol Infect 2014; 20(suppl 7):19–26. [DOI] [PubMed] [Google Scholar]
- 30. Chan ST, Logan AC. The clinical impact of cytomegalovirus infection following allogeneic hematopoietic cell transplantation: why the quest for meaningful prophylaxis still matters. Blood Rev 2017; 31:173–83. [DOI] [PubMed] [Google Scholar]
- 31. Plotkin SA. et al. . The status of vaccine development against the human cytomegalovirus. J Infect Dis 2019; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krause PR, Bialek SR, Boppana SB, et al. . Priorities for CMV vaccine development. Vaccine 2013; 32:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lanzieri TM. et al. . Mathematical models of vaccination for preventing congenital cytomegalovirus infection. J Infect Dis 2019; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]