Abstract
Somatic RUNX1 mutations are found in approximately 10% of patients with de novo acute myeloid leukemia (AML), but are more common in secondary forms of myelodysplastic syndrome (MDS) or AML. Particularly, this applies to MDS/AML developing from certain types of leukemia-prone inherited bone marrow failure syndromes. How these RUNX1 mutations contribute to the pathobiology of secondary MDS/AML is still unknown. This mini-review focusses on the role of RUNX1 mutations as the most common secondary leukemogenic hit in MDS/AML evolving from severe congenital neutropenia (SCN).
Keywords: leukemic progression, RUNX1, severe congenital neutropenia
INTRODUCTION
The occurrence and frequency of RUNX1 mutations in a variety of hematological malignancies has been well-documented (Sood et al., 2017). Originally identified as a chromosomal translocation partner in the so-called core-binding factor (CBF) leukemias, somatic RUNX1 mutations were also found in myeloid malignancies, particularly in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) (Chen et al., 2007; Christiansen et al., 2004; Gaidzik et al., 2011; Harada et al., 2004; Mangan and Speck, 2011; Osato, 2004; Schnittger et al., 2011; Steensma et al., 2005; Tang et al., 2009). Somatic mutations in RUNX1 cluster mostly within the N-terminal Runt homology domain (RHD) whereas mutations disrupting the C-terminal transactivation domain (TAD) occur less frequently (Gaidzik et al., 2011; Preudhomme et al., 2000; Schnittger et al., 2011; Tang et al., 2009). Importantly, mutations in RUNX1 were identified as the cause of familial platelet disorder, in which patients show a predisposition to develop MDS or AML (FPDMM or FPD/AML) (Song et al., 1999). These germline mutations are similar to those acquired in MDS/AML (Song et al., 1999). Finally, it has become clear that somatic RUNX1 mutations are particularly prevalent in MDS/AML secondary to inherited bone marrow failure syndromes (iBMFs) such as Fanconi anemia and severe congenital neutropenia (SCN), and in radiation-associated MDS/AML (Harada et al., 2003; Quentin et al., 2011; Skokowa et al., 2014). These forms of secondary MDS/AML (sMDS/AML) are characterized by an adverse prognosis due to refractoriness to treatment. Why secondary RUNX1 mutations are associated with sMDS/AML and how they contribute to the pathogenesis of these conditions remains largely unclear. Here, we will discuss the current insights and ideas regarding mutant RUNX1 in the context of malignant transformation of iBMFs, taking SCN as the leading example. Specifically, we will briefly summarize and discuss our most recent insights into these issues based on observations in patients, mouse- and induced pluripotent stem cell (iPSC)-models.
SEVERE CONGENITAL NEUTROPENIA
SCN is an iBMF characterized by severely reduced neutrophil counts, leading to life-threatening bacterial infections (Skokowa et al., 2017). Autosomal dominant mutations in ELANE, the gene encoding neutrophil elastase, are the most frequently observed genetic defects in SCN patients. How these mutations give rise to severe neutropenia is still largely unknown (Skokowa et al., 2017). Life-long administration of colony stimulating factor 3 (CSF3), also known as granulocyte colony-stimulating factor (G-CSF), successfully alleviates the neutropenia in the majority of SCN patients (Dale et al., 1993). Importantly, SCN patients have a high risk of developing MDS or AML, with a median incidence of 21%, 15 years after initiation of CSF3 treatment (Rosenberg et al., 2006; 2010). The majority of SCN patients with leukemic progression show the appearance of hematopoietic clones with somatic mutations in CSF3R, resulting in a truncated form of CSF3R with defective internalization and aberrant signaling properties (Touw, 2015). These clones may persist for months or even years before MDS or AML becomes overt (Germeshausen et al., 2007), raising the question how these CSF3R mutants contribute to the malignant transformation of SCN. Activation of oxidative stress through enhanced production of reactive oxygen species (ROS) and sustained activation of signal transducer and activator of transcription STAT5 have been put forward as candidate mechanisms by which activation of truncated CSF3R drive clonal expansion of myeloid progenitors (Liu et al., 2008; Zhu et al., 2006).
RUNX1 MUTATIONS IN SCN-MDS/AML PATIENTS
Like for numerous other disease conditions, the introduction of massive parallel (“next generation”) sequencing has greatly advanced our insights into the genomic defects associated with the leukemic progression of SCN. A retrospective analysis in an ELANE-SCN patient, who continuously received CSF3 therapy for 15 years and during which period serial BM sampling was done, showed that after the occurrence of multiple CSF3R mutant clones 2 years after the start of CSF3 treatment, no additional mutations were detected until MDS/AML became clinically overt (Beekman et al., 2012). At that fully transformed stage, a limited number of clonal mutations in regulatory genes, including RUNX1, SUZ12, ASXL1 and EP300, were present (Beekman et al., 2012). This pattern of leukemic evolution was confirmed in a follow-up study involving 31 SCN-MDS/AML cases (Skokowa et al., 2014). Importantly, this study revealed that mutations in RUNX1 are by far the most frequent somatic secondary mutations in SCN-MDS/AML and preferentially occurred in CSF3R mutation clones. In SCN-MDS/AML, mainly RUNX1 mutations disrupting the RHD, essential for DNA binding and for interaction with the regulatory protein CBFb, were found (Beekman et al., 2012; Skokowa et al., 2014). In view of these characteristics, the molecular pathogenesis of SCN/AML serves as an attractive model to investigate the role of secondary RUNX1 mutations in a molecularly well-defined process of leukemic progression.
MOUSE MODEL TO STUDY THE IMPACT OF Csf3r AND RUNX1 MUTATIONS IN CONJUNCTION WITH CSF3 TREATMENT
The impact of Runx1 and mutants on hematopoietic cell development has been investigated in a variety of mouse models and has been the subject of several recent reviews (Bellissimo and Speck, 2017; Chin et al., 2015; Harada and Harada, 2009; Sood et al., 2017). Notwithstanding some contradictory results, possibly related to discrepancies in the immune-phenotyping based classification of stem cell subpopulations, it is generally accepted that wild type Runx1 has no major impact on the production and function of long-term hematopoietic stem cells in mice, both under homeostatic conditions and under conditions of proliferative stress (Cai et al., 2011). More relevant in the context of RUNX1 mutations in SCN-MDS/AML are the mouse models with RUNX1-RHD mutations equivalent to those recurrently found in patients (Harada et al., 2004). Watanabe-Okochi and colleagues studied the effects of such a mutant in transplantation experiments, in which donor bone marrow (BM) cells were transduced with a murine leukemia virus (MLV)-derived vector to express the most common RUNX1 mutant D171N and reported that this resulted in MDS and MDS/AML (Watanabe-Okochi et al., 2008). However, integration of the MLV-based vector in the Mecom (Evi1) locus caused overexpression of Evi1 in these mice (Watanabe-Okochi et al., 2008). Because the combination of RUNX1 mutations and high EVI1 expression is rarely seen in MDS/AML, and because high Evi1 expression can be leukemogenic by itself, the contribution of RUNX1-RHD in MDS/AML development in more general could not be accurately deduced from this model (Harada et al., 2013; Watanabe-Okochi et al., 2008). In fact, expression of mutant D171N in human cord blood cells had marginal effects on the rate of proliferation and differentiation capacity of CD34+ cells in in vitro suspension culture relative to empty vector control cells, suggesting that isolated RUNX-RHD mutations are only weakly leukemogenic (Goyama et al., 2013).
To study the role of RUNX1-D171N in a context relevant to SCN-MDS/AML, we used a mouse model expressing a truncated Csf3r (Csf3r-d715) identical to the mutant CSF3R form in SCN patients (Hermans et al., 1998; 1999). To avoid the tropism of MLV-based vectors for oncogenic enhancers, we generated a lentiviral expression vector to express RUNX1 mutant D171N (Goyama et al., 2013) in conjunction with enhanced green fluorescent protein (eGFP) in Csf3r-d715 BM cells, which were subsequently serially transplanted in wild type recipients. Recipients were treated either 3× a week with CSF3 or with PBS (solvent control). Transcriptome analysis and whole exome sequencing on FACS purified eGFP+Lin–c-Kit+ (LK) populations were done to identify molecular pathways associated with leukemic progression. Sequential CD34+ cell samples from a SCN/AML patient with identical CSF3R and RUNX1 mutations (Beekman et al., 2012) and whole genome sequencing data from diagnostic AML samples were used for clinical comparisons (Olofsen et al., 2018).
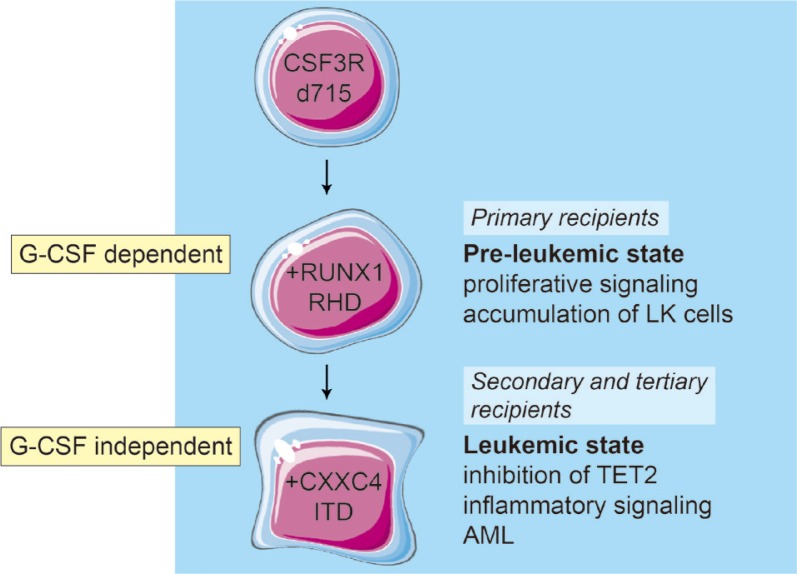
CSF3 treatment of primary recipients transplanted with Csf3r-RUNX1 mutant BM cells resulted in sustained (30+ weeks) presence of eGFP+LK cells in the peripheral blood (PB), which had the morphological appearance of myeloblasts. The PB also contained eGFP+ neutrophils, indicating that myeloid differentiation was not completely blocked. Importantly, none of these primary recipient mice succumbed to symptoms of AML, suggesting that the elevated myeloblasts in the PB reflected a pre-leukemic rather than a fully transformed state. However, upon transplantation in secondary and tertiary recipients, mice developed Csf3r-RUNX1 mutant AML that was no longer dependent on CSF3 administration. Transcriptome profiles of purified eGFP+LK cells sorted before transplantation, showed that expression of RUNX1 mutant protein in Csf3r mutant cells resulted in elevated proliferative/metabolic signatures characterized by elevated MYC and mTORC1 signaling relative to empty vector controls. Strikingly, at the sequential steps of leukemic transformation in the mouse model, these signatures declined while TNFa-, interferon- and interleukin-6–driven inflammatory responses were increasingly upregulated. Whole exome sequencing performed on the LK-cells from these stages revealed that an internal tandem duplication (ITD) in Cxxc4 was acquired. In the secondary and tertiary recipients all AML cells harbored the Csf3r, RUNX1 and heterozygous Cxxc4 mutations, while the primary recipient showed a subclonal Cxxc4 mutation (VAF: 0.27). The mutation resulted in a 7-fold higher expression of CXXC4 protein. CXXC4 was previously shown to inhibit TET2 protein levels (Hino et al., 2001; Ko et al., 2013) and in agreement with this, TET2 levels were strongly reduced in the CXXC4 mutant/overexpressing leukemic samples. Intriguingly, CXXC4 mutations have also been detected in human AML cases, including the ITD mutations identified in our mouse model (Olofsen et al., 2018; Olofsen et al., Unpublished reference). These observations in mice fit into a model in which the activation of a truncated Csf3r by the sustained administration of CSF3 and the presence of RUNX1-RHD mutant D171N give rise to a premalignant state, characterized by the accumulation of LK cells in the PB and elevated activation of proliferative signalling (Fig. 1). An additional clonal mutation that reduces the levels of TET2 drives the full transformation to AML, at which stage the leukemia-initiating cells have lost their need for CSF3 for propagation in vivo and the AML blasts have adopted an inflammatory signature identical to that of SCN/AML cells with identical mutations in CSF3R and RUNX1 (Fig. 1) (Beekman et al., 2012; Schmied et al., Unpublished reference). Although CXXC4 mutations have thus far not been reported in clinical SCN/AML samples, mutations potentially affecting TET2 levels and/or function, such as mutations in polycomb repressor complex-2 genes (EZH2, SUZ12) are recurrently present (Beekman et al., 2012; Skokowa et al., 2014).
Fig. 1. Model of leukemic progression in mice based on serial transplantation of Csf3r-d715/RUNX1-RHD mutant BM cells.
In primary recipients, the combination of Csf3r-d715 and RUNX1-D171N gives rise to accumulation of immature LK cells. This occurs only when mice are treated with G-CSF (CSF3) and mice do not succumb to symptoms of leukemia. Upon secondary and subsequent transplantations of these LK cells, a G-CSF independent AML develops, which is characterized by elevated inflammatory responses and reduced TET2 protein levels.
STUDIES IN INDUCED PLURIPOTENT STEM CELL (iPSC) MODELS
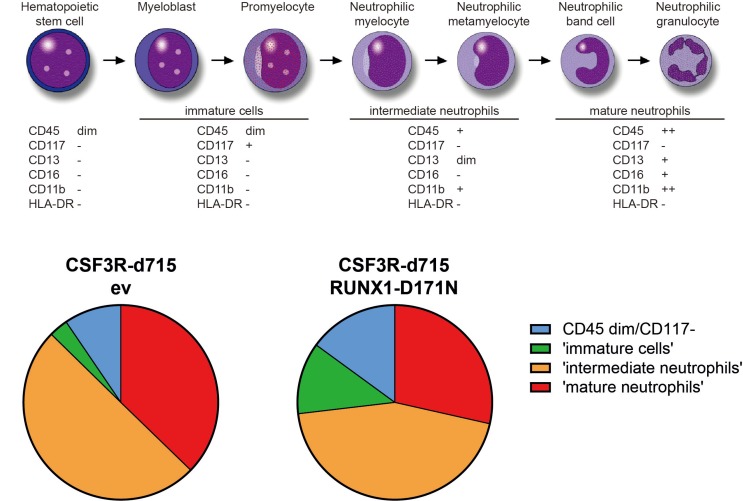
The use of patient-derived iPSC lines has created new possibilities to model diseases, including myeloid malignancies (Papapetrou, 2019). In the context of RUNX1, these studies have mainly dealt with FPD/AML, characterized by germline RUNX1 mutations (Antony-Debre et al., 2015; Connelly et al., 2014; Sakurai et al., 2014). Key features of these iPSC lines are (i) their reduced ability to generate CD34+CD45+ hematopoietic stem and progenitor cells (HSPCs) and (ii) their affected ability of megakaryocyte (Mk) production and pro-platelet formation, thus explaining the platelet defects observed in patients. The reduced production of HSPCs from FPD-derived iPSCs is consistent with a role of RUNX1 in hematopoietic development from pluripotent stem cells (Yzaguirre et al., 2017). As mentioned above, in SCN patients who develop MDS or AML, RUNX1 mutations are most often acquired in CSF3R mutant HSPC clones. Hence, it is important in this context to assess the consequences of somatic RUNX1 mutations in HSPCs cells that already harbor a CSF3R nonsense mutation. To achieve this, a CRISPR/Cas9-based strategy was used to introduce a patient-derived CSF3R nonsense mutation into iPSCs. After switching the cells to hematopoietic culture conditions (STEMdiff Hematopoietic Kit from STEMCELL Technologies, Canada), CD34+CD45+ cells were lentivirally transduced to express the RUNX1-RHD D171N mutant. These experiments showed that the combined presence of CSF3R and RUNX1 mutations had a moderate effect on myeloid differentiation, characterized by a relative abundance of immature neutrophilic differentiation stages, but not by an absolute differentiation block (Fig. 2). As such, these findings corroborate the findings in the mouse model described above and further suggest that secondary RUNX1 mutations in clones with CSF3R mutations do not confer a fully transformed, i.e., MDS/AML like phenotype. In agreement with this, transcriptome analysis showed that the CSF3R-RUNX1 mutant cells had elevated proliferative signatures but did not show the inflammatory profiles seen in the SCN/AML patient and the mouse AML cells. A key question that remains to be addressed is how mutations in ELANE, HAX1 and other SCN-causing mutations contribute to leukemic progression in conjunction with CSF3R and RUNX1 mutations. Preliminary data from these models suggest that ELANE and HAX1 mutations cause elevated levels of ROS in CD34+CD45+ HSPCs generated from SCN-iPSCs, resulting in the upregulation of anti-oxidant pathways (Olofsen et al., 2019). Future work should clarify whether and to what extent the oxidative damage caused by ROS and the adaptive anti-oxidant protection mechanisms contribute to malignant transformation.
Fig. 2. Myeloid differentiation of iPSC-derived CD34+CD45+ cells, genome-edited to express a truncated form (d715) of CSF3R and transduced with RUNX1-D171N lentiviral expression vector or empty vector (ev) control.
Cells were cultured in suspension for a total of 9 days in medium supplemented with myeloid growth factors, i.e., a cocktail of IL3, SCF, GM-CSF and G-CSF for the first 4 days, followed by G-CSF as the single growth factor for the next 5 days.
CONCLUSIONS AND OUTLOOK
The role of RUNX1 mutations in the development of MDS and AML remains incompletely understood. Studies in mouse-, patient- and iPSC-models, addressing the role of a recurrent RUNX1 mutation (D171N) in combination with the most frequent CSF3R mutation in the leukemic progression of SCN (CSF3R-d715), showed that RUNX1-D171N enhanced the activation of proliferative signaling pathways but only mildly affected myeloid differentiation, leading to a relative accumulation of immature cells but not to an absolute differentiation block. Furthermore, the studies in mice showed that the combination of these two mutations is not enough for leukemic progression, even when the mice were subjected to sustained G-CSF (CSF3) treatment. These findings established that additional events, one of which affecting TET2 levels, are necessary for full leukemic transformation. Leukemic progression in all models was also associated with enhanced interferon-g, interleukin-6 and TNFa/NFkB signaling, suggesting that inflammation is a major additional component in the development of myeloid malignancy involving RUNX1 mutations. How this interplay between mutant CSF3R signaling, aberrant transcriptional control by mutant RUNX1, loss of TET2 function and inflammatory responses contributes to leukemic transformation and what the exact causal relationships between these mechanisms are remains to be addressed. Detailed insights into this complex network of events may help to discover biomarkers for early detection of leukemic progression of SCN and possibly other forms of iBMFs and may provide leads for novel forms of therapeutic intervention to avoid full malignant transformation of these conditions.
ACKNOWLEDGMENTS
This work was financially supported by grants from the Dutch Cancer Society “KWF-kankerbestrijding”.
Footnotes
Disclosure
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Antony-Debre I., Manchev V.T., Balayn N., Bluteau D., Tomowiak C., Legrand C., Langlois T., Bawa O., Tosca L., Tachdjian G., et al. Level of RUNX1 activity is critical for leukemic predisposition but not for thrombocytopenia. Blood. 2015;125:930–940. doi: 10.1182/blood-2014-06-585513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman R., Valkhof M.G., Sanders M.A., van Strien P.M., Haanstra J.R., Broeders L., Geertsma-Kleinekoort W.M., Veerman A.J., Valk P.J., Verhaak R.G., et al. Sequential gain of mutations in severe congenital neutropenia progressing to acute myeloid leukemia. Blood. 2012;119:5071–5077. doi: 10.1182/blood-2012-01-406116. [DOI] [PubMed] [Google Scholar]
- Bellissimo D.C., Speck N.A. RUNX1 mutations in inherited and sporadic leukemia. Front. Cell Dev. Biol. 2017;5:111. doi: 10.3389/fcell.2017.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Gaudet J.J., Mangan J.K., Chen M.J., De Obaldia M.E., Oo Z., Ernst P., Speck N.A. Runx1 loss minimally impacts long-term hematopoietic stem cells. PLoS One. 2011;6:e28430. doi: 10.1371/journal.pone.0028430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Y., Lin L.I., Tang J.L., Ko B.S., Tsay W., Chou W.C., Yao M., Wu S.J., Tseng M.H., Tien H.F. RUNX1 gene mutation in primary myelodysplastic syndrome--the mutation can be detected early at diagnosis or acquired during disease progression and is associated with poor outcome. Br. J. Haematol. 2007;139:405–414. doi: 10.1111/j.1365-2141.2007.06811.x. [DOI] [PubMed] [Google Scholar]
- Chin D.W., Watanabe-Okochi N., Wang C.Q., Tergaonkar V., Osato M. Mouse models for core binding factor leukemia. Leukemia. 2015;29:1970–1980. doi: 10.1038/leu.2015.181. [DOI] [PubMed] [Google Scholar]
- Christiansen D.H., Andersen M.K., Pedersen-Bjergaard J. Mutations of AML1 are common in therapy-related myelodysplasia following therapy with alkylating agents and are significantly associated with deletion or loss of chromosome arm 7q and with subsequent leukemic transformation. Blood. 2004;104:1474–1481. doi: 10.1182/blood-2004-02-0754. [DOI] [PubMed] [Google Scholar]
- Connelly J.P., Kwon E.M., Gao Y., Trivedi N.S., Elkahloun A.G., Horwitz M.S., Cheng L., Liu P.P. Targeted correction of RUNX1 mutation in FPD patient-specific induced pluripotent stem cells rescues megakaryopoietic defects. Blood. 2014;124:1926–1930. doi: 10.1182/blood-2014-01-550525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale D.C., Bonilla M.A., Davis M.W., Nakanishi A.M., Hammond W.P., Kurtzberg J., Wang W., Jakubowski A., Winton E., Lalezari P., et al. A randomized controlled phase III trial of recombinant human granulocyte colony-stimulating factor (filgrastim) for treatment of severe chronic neutropenia. Blood. 1993;81:2496–2502. doi: 10.1182/blood.V81.10.2496.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidzik V.I., Bullinger L., Schlenk R.F., Zimmermann A.S., Röck J., Paschka P., Corbacioglu A., Krauter J., Schlegelberger B., Ganser A., et al. RUNX1 mutations in acute myeloid leukemia: results from a comprehensive genetic and clinical analysis from the AML study group. J. Clin. Oncol. 2011;29:1364–1372. doi: 10.1200/JCO.2010.30.7926. [DOI] [PubMed] [Google Scholar]
- Germeshausen M., Ballmaier M., Welte K. Incidence of CSF3R mutations in severe congenital neutropenia and relevance for leukemogenesis: results of a long-term survey. Blood. 2007;109:93–99. doi: 10.1182/blood-2006-02-004275. [DOI] [PubMed] [Google Scholar]
- Goyama S., Schibler J., Cunningham L., Zhang Y., Rao Y., Nishimoto N., Nakagawa M., Olsson A., Wunderlich M., Link K.A., et al. Transcription factor RUNX1 promotes survival of acute myeloid leukemia cells. J. Clin. Invest. 2013;123:3876–3888. doi: 10.1172/JCI68557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H., Harada Y., Niimi H., Kyo T., Kimura A., Inaba T. High incidence of somatic mutations in the AML1/RUNX1 gene in myelodysplastic syndrome and low blast percentage myeloid leukemia with myelodysplasia. Blood. 2004;103:2316–2324. doi: 10.1182/blood-2003-09-3074. [DOI] [PubMed] [Google Scholar]
- Harada H., Harada Y., Tanaka H., Kimura A., Inaba T. Implications of somatic mutations in the AML1 gene in radiation-associated and therapy-related myelodysplastic syndrome/acute myeloid leukemia. Blood. 2003;101:673–680. doi: 10.1182/blood-2002-04-1010. [DOI] [PubMed] [Google Scholar]
- Harada Y., Harada H. Molecular pathways mediating MDS/AML with focus on AML1/RUNX1 point mutations. J. Cell. Physiol. 2009;220:16–20. doi: 10.1002/jcp.21769. [DOI] [PubMed] [Google Scholar]
- Harada Y., Inoue D., Ding Y., Imagawa J., Doki N., Matsui H., Yahata T., Matsushita H., Ando K., Sashida G., et al. RUNX1/AML1 mutant collaborates with BMI1 overexpression in the development of human and murine myelodysplastic syndromes. Blood. 2013;121:3434–3446. doi: 10.1182/blood-2012-06-434423. [DOI] [PubMed] [Google Scholar]
- Hermans M.H., Antonissen C., Ward A.C., Mayen A.E., Ploemacher R.E., Touw I.P. Sustained receptor activation and hyperproliferation in response to granulocyte colony-stimulating factor (G-CSF) in mice with a severe congenital neutropenia/acute myeloid leukemia-derived mutation in the G-CSF receptor gene. J. Exp. Med. 1999;189:683–692. doi: 10.1084/jem.189.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans M.H., Ward A.C., Antonissen C., Karis A., Lowenberg B., Touw I.P. Perturbed granulopoiesis in mice with a targeted mutation in the granulocyte colony-stimulating factor receptor gene associated with severe chronic neutropenia. Blood. 1998;92:32–39. doi: 10.1182/blood.V92.1.32.413k42_32_39. [DOI] [PubMed] [Google Scholar]
- Hino S., Kishida S., Michiue T., Fukui A., Sakamoto I., Takada S., Asashima M., Kikuchi A. Inhibition of the Wnt signaling pathway by Idax, a novel Dvl-binding protein. Mol. Cell. Biol. 2001;21:330–342. doi: 10.1128/MCB.21.1.330-342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M., An J., Bandukwala H.S., Chavez L., Aijö T., Pastor W.A., Segal M.F., Li H., Koh K.P., Lähdesmäki H., et al. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature. 2013;497:122–126. doi: 10.1038/nature12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Kunter G., Krem M.M., Eades W.C., Cain J.A., Tomasson M.H., Hennighausen L., Link D.C. Csf3r mutations in mice confer a strong clonal HSC advantage via activation of Stat5. J. Clin. Invest. 2008;118:946–955. doi: 10.1172/JCI32704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan J.K., Speck N.A. RUNX1 mutations in clonal myeloid disorders: from conventional cytogenetics to next generation sequencing, a story 40 years in the making. Crit. Rev. Oncog. 2011;16:77–91. doi: 10.1615/CritRevOncog.v16.i1-2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsen P.A., Fatrai S., van Strien P.M.H., Obenauer J.C., Hoogenboezem R.M., Erpelinck-Verschueren C.A.J., Roovers O., Haferlach T., Valk P., Schneider R.K., et al. A leukemic progression model of severe congenital neutropenia uncovers a novel mechanism of AML development involving elevated inflammatory responses, mutation of CXXC4 and decreased TET2 levels. Blood. 2018;132:540. doi: 10.1182/blood-2018-99-115914. [DOI] [Google Scholar]
- Olofsen P.A., van Strien P.M.H., Roovers O., de Looper H.W.J., Hoogenboezem R.M., Bosch D.A., Ghazvini M., Bindels E.M.J., de Pater E.M., Touw I.P. PML plays a key role in severe congenital neutropenia with mutant elane causing neutrophil elastase protein misfolding. Blood. 2019;134:213. doi: 10.1182/blood-2019-122423. [DOI] [Google Scholar]
- Osato M. Point mutations in the RUNX1/AML1 gene: another actor in RUNX leukemia. Oncogene. 2004;23:4284–4296. doi: 10.1038/sj.onc.1207779. [DOI] [PubMed] [Google Scholar]
- Papapetrou E.P. Modeling myeloid malignancies with patient-derived iPSCs. Exp. Hematol. 2019;71:77–84. doi: 10.1016/j.exphem.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preudhomme C., Warot-Loze D., Roumier C., Grardel-Duflos N., Garand R., Lai J.L., Dastugue N., Macintyre E., Denis C., Bauters F., et al. High incidence of biallelic point mutations in the Runt domain of the AML1/PEBP2 alpha B gene in Mo acute myeloid leukemia and in myeloid malignancies with acquired trisomy 21. Blood. 2000;96:2862–2869. doi: 10.1182/blood.V96.8.2862. [DOI] [PubMed] [Google Scholar]
- Quentin S., Cuccuini W., Ceccaldi R., Nibourel O., Pondarre C., Pagès M.P., Vasquez N., Dubois d'Enghien C., Larghero J., Peffault, de Latour R., et al. Myelodysplasia and leukemia of Fanconi anemia are associated with a specific pattern of genomic abnormalities that includes cryptic RUNX1/AML1 lesions. Blood. 2011;117:e161–e170. doi: 10.1182/blood-2010-09-308726. [DOI] [PubMed] [Google Scholar]
- Rosenberg P.S., Alter B.P., Bolyard A.A., Bonilla M.A., Boxer L.A., Cham B., Fier C., Freedman M., Kannourakis G., Kinsey S., et al. The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood. 2006;107:4628–4635. doi: 10.1182/blood-2005-11-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg P.S., Zeidler C., Bolyard A.A., Alter B.P., Bonilla M.A., Boxer L.A., Dror Y., Kinsey S., Link D.C., Newburger P.E., et al. Stable long-term risk of leukaemia in patients with severe congenital neutropenia maintained on G-CSF therapy. Br. J. Haematol. 2010;150:196–199. doi: 10.1111/j.1365-2141.2010.08216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai M., Kunimoto H., Watanabe N., Fukuchi Y., Yuasa S., Yamazaki S., Nishimura T., Sadahira K., Fukuda K., Okano H., et al. Impaired hematopoietic differentiation of RUNX1-mutated induced pluripotent stem cells derived from FPD/AML patients. Leukemia. 2014;28:2344–2354. doi: 10.1038/leu.2014.136. [DOI] [PubMed] [Google Scholar]
- Schnittger S., Dicker F., Kern W., Wendland N., Sundermann J., Alpermann T., Haferlach C., Haferlach T. RUNX1 mutations are frequent in de novo AML with noncomplex karyotype and confer an unfavorable prognosis. Blood. 2011;117:2348–2357. doi: 10.1182/blood-2009-11-255976. [DOI] [PubMed] [Google Scholar]
- Skokowa J., Dale D.C., Touw I.P., Zeidler C., Welte K. Severe congenital neutropenias. Nat. Rev. Dis. Primers. 2017;3:17032. doi: 10.1038/nrdp.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokowa J., Steinemann D., Katsman-Kuipers J.E., Zeidler C., Klimenkova O., Klimiankou M., Unalan M., Kandabarau S., Makaryan V., Beekman R., et al. Cooperativity of RUNX1 and CSF3R mutations in severe congenital neutropenia: a unique pathway in myeloid leukemogenesis. Blood. 2014;123:2229–2237. doi: 10.1182/blood-2013-11-538025. [DOI] [PubMed] [Google Scholar]
- Song W.J., Sullivan M.G., Legare R.D., Hutchings S., Tan X., Kufrin D., Ratajczak J., Resende I.C., Haworth C., Hock R., et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat. Genet. 1999;23:166–175. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- Sood R., Kamikubo Y., Liu P. Role of RUNX1 in hematological malignancies. Blood. 2017;129:2070–2082. doi: 10.1182/blood-2016-10-687830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensma D.P., Gibbons R.J., Mesa R.A., Tefferi A., Higgs DR. Somatic point mutations in RUNX1/CBFA2/AML1 are common in high-risk myelodysplastic syndrome, but not in myelofibrosis with myeloid metaplasia. Eur. J. Haematol. 2005;74:47–53. doi: 10.1111/j.1600-0609.2004.00363.x. [DOI] [PubMed] [Google Scholar]
- Tang J.L., Hou H.A., Chen C.Y., Liu C.Y., Chou W.C., Tseng M.H., Huang C.F., Lee F.Y., Liu M.C., Yao M., et al. AML1/RUNX1 mutations in 470 adult patients with de novo acute myeloid leukemia: prognostic implication and interaction with other gene alterations. Blood. 2009;114:5352–5361. doi: 10.1182/blood-2009-05-223784. [DOI] [PubMed] [Google Scholar]
- Touw I.P. Game of clones: the genomic evolution of severe congenital neutropenia. Hematology Am. Soc. Hematol. Educ. Program. 2015;2015:1–7. doi: 10.1182/asheducation-2015.1.1. [DOI] [PubMed] [Google Scholar]
- Watanabe-Okochi N., Kitaura J., Ono R., Harada H., Harada Y., Komeno Y., Nakajima H., Nosaka T., Inaba T., Kitamura T. AML1 mutations induced MDS and MDS/AML in a mouse BMT model. Blood. 2008;111:4297–4308. doi: 10.1182/blood-2007-01-068346. [DOI] [PubMed] [Google Scholar]
- Yzaguirre A.D., de Bruijn M.F., Speck N.A. The role of Runx1 in embryonic blood cell formation. Adv. Exp. Med. Biol. 2017;962:47–64. doi: 10.1007/978-981-10-3233-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q.S., Xia L., Mills G.B., Lowell C.A., Touw I.P., Corey S.J. G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood. 2006;107:1847–1856. doi: 10.1016/j.ebiom.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]