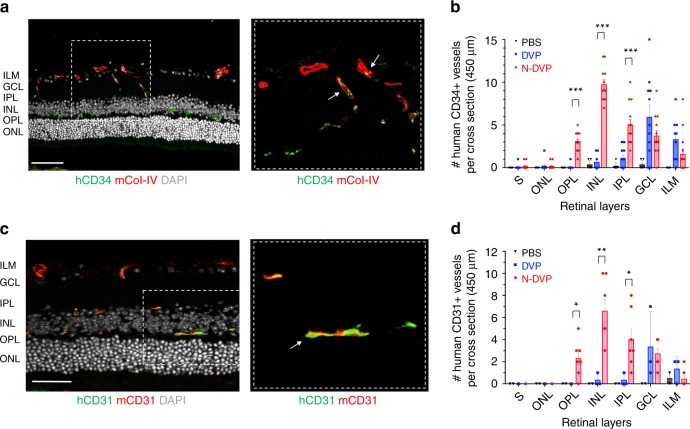
Fig. 7. Migration and vascular engraftment of primed vs. naïve DVP into ischemia-injured blood vessels of the neural retina.
Cross-sectioned retinae of I/R-injured eyes of NOG mice were immunostained with either human CD34 (a, b; hCD34) or human CD31 (c, d; hCD31) antibodies 2 weeks following DVP vs. N-DVP injections. Antibodies for murine collagen type-IV (mCol-IV) were also employed to detect murine blood vessel basement membrane, and murine CD31 (mCD31) detected murine endothelium. The number of b human CD34+ or d human CD31+ cells detected within transverse layers of the murine neural retina (per 450 μm retinal cross section) was quantitated with imaging software. Each data point represents a replicate individual 450 μm retinal cross section that was analyzed from I/R- treated eyes injected with saline (PBS), primed DVP or N-DVP. Human CD34+ or human CD31+ endothelial cell engraftment was enumerated in each distinct layer of neural retina shown; only N-DVP migrated into the inner nuclear layer (INL) while most of the primed DVP remained primarily in the superficial ganglion cell layer (GCL). ILM: inner limiting membrane, IPL: inner plexiform layer, outer nuclear layer (ONL), OPL: outer plexiform layer, S: segments. All scale bars = 50 μm. Each individual quantitation shown is an independent experimental measurement with (standard error of mean) SEM from an individually-immunostained cryosection for each of the three groups of injected mouse eyes (i.e., saline-PBS (n = 8), DVP (n = 3, CD31; n = 11, CD34), N-DVP (n = 7, CD31; n = 14, CD34). ***p < 0.001; **p < 0.01; *p < 0.05 (multiple unpaired t-tests).