Abstract
Background
In an effort to improve outcomes of in‐vitro fertilisation cycles the use of growth hormone has been considered. Improving the outcomes of in‐vitro fertilisation is especially important for subfertile women who are considered 'poor responders'.
Objectives
To assess the effectiveness of adjuvant growth hormone in in‐vitro fertilisation protocols.
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Groups trials register (June 2009), the Cochrane Central Register of Controlled Trials (Cochrane Library Issue 2, 2009), MEDLINE (1966 to June 2009), EMBASE (1988 to June 2009) and Biological Abstracts (1969 to June 2009).
Selection criteria
All randomised controlled trials were included if they addressed the research question and provided outcome data for intervention and control participants.
Data collection and analysis
Assessment of trial risk of bias and extraction of relevant data was performed independently by two reviewers.
Main results
Ten studies (440 subfertile couples) were included. Results of the meta‐analysis demonstrated no difference in outcome measures and adverse events in the routine use of adjuvant growth hormone in in‐vitro fertilisation protocols. However, meta‐analysis demonstrated a statistically significant difference in both live birth rates and pregnancy rates favouring the use of adjuvant growth hormone in in‐vitro fertilisation protocols in women who are considered poor responders without increasing adverse events, OR 5.39, 95% CI 1.89 to 15.35 and OR 3.28, 95% CI 1.74 to 6.20 respectively.
Authors' conclusions
Although the use of growth hormone in poor responders has been found to show a significant improvement in live birth rates, we were unable to identify which sub‐group of poor responders would benefit the most from adjuvant growth hormone. The result needs to be interpreted with caution, the included trials were few in number and small sample size. Therefore, before recommending growth hormone adjuvant in in‐vitro fertilisation further research is necessary to fully define its role.
Plain language summary
Growth hormone in in‐vitro fertilisation
Before starting an in‐vitro fertilisation cycle, some women need help to ovulate and the use of growth hormone therapy may help these women. This aims to reduce the use of gonadotropin therapy to stimulate ovulation, a hormone that can cause multiple pregnancy. The review of trials found no evidence that growth hormone helps improve birth rates in women who are undergoing ovulation induction prior to in‐vitro fertilisation. However there is some evidence of increased pregnancy and birth rates in women who are considered 'poor responders' to in‐vitro fertilisation. More research is needed.
Background
Description of the condition
Subfertility, usually defined as absence of conception after one year of regular intercourse, is a common problem affecting as many as one in six couples (Cahill 2002). Main causes include sperm dysfunction, ovulation disorder and fallopian tube damage (Cahill 2002). One method of treating infertile couples is assisted conception via in‐vitro fertilisation (IVF). IVF involves using hormones to modify ovarian function in order to increase follicular growth and thus develop more than one oocyte. Ovulation is then triggered with human chorionic gonadotropin and the oocytes are retrieved and fertilised with sperm in the laboratory setting. The oocytes are then fertilised outside the body (in vitro). The fertilised oocytes (embryos) are then transferred into the uterus after 36 hours after oocyte retrieval. IVF protocols are constantly under review in an attempt to decrease hormone (gonadotrophin) requirement, improve follicular recruitment, whilst primarily to increase live birth rates.
Description of the intervention
Some protocols have considered the role of growth hormone in IVF (Landolfi 1994; Jacobs 1995). Growth hormone is a biological peptide hormone, synthesised, stored and secreted by somatotroph cells located in the anterior pituitary gland. Growth hormone can be synthetically produced using recombinant DNA technology and is licensed to be used in the human population. There is currently no consensus as to the route, dose or timing of growth hormone administration in IVF protocols.
How the intervention might work
The administration of growth hormone may potentate the effect of exogenous gonadotrophins (Homburg 1988). Growth hormone is reported to modulate the action of follicular stimulating hormone on granulosa cells by up‐regulating the local synthesis of insulin‐like growth factor‐I (IGF‐1). This interest has been stimulated by animal studies which suggest that growth hormone may increase the intra‐ovarian production of the IGF‐1 (Hsu 1987; Yoshimura 1996). IGF‐1 displays growth hormone dependence both in‐vivo and in‐vitro (Blumenfeld 1996). The interaction between growth hormone and IGF‐1 is of significance since IGF‐1 has been shown to play an important part in ovarian function in both animal and human models (Adashi 1985; Erickson 1989).The addition of IGF‐1 to gonadotrophins in granulosa cell cultures increased gonadotrophin action on the ovary by several mechanisms including augmentation of aromatase activity, 17 beta‐oestradiol and progesterone production and luteinising hormone receptor formation (Erickson 1989;Mason 1990). IGF‐1 has also been found to stimulate follicular development, oestrogen production and oocyte maturation (Yoshimura 1996).
Why it is important to do this review
Improving the outcomes of IVF by the use of growth hormone adjuvant therapy is important particularly in those women who are considered poor responders. The aim of this review is to establish the role of growth hormone in IVF.
Objectives
To assess the effectiveness of adjuvant growth hormone in IVF.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials were eligible for inclusion.
Types of participants
Women who were part of a subfertile couple undergoing IVF.
Types of interventions
All studies comparing adjuvant growth hormone in IVF cycles with standard IVF cycles.
Types of outcome measures
Primary outcomes
1. Live birth rate per woman randomised
Number of women achieving a live birth divided by the number of women randomised.
Secondary outcomes
1. Pregnancy rate per woman randomised.
Number of women achieving a clinical pregnancy (established with a human chorionic gonadotropin test in blood or urine and/or confirmed by ultrasound) divided by the number of women randomised.
2. Oocytes retrieved per woman randomised.
Number of women with at least one oocyte retrieved divided by the number of women randomised.
3. Embryo transfer per woman randomised.
Number of women with at least one embryo transferred divided by the number of women randomised.
4. Ampoules of gonadotrophin used.
The mean number of ampoules used per woman.
5. Adverse events (e.g. ovarian hyperstimulation syndrome).
Search methods for identification of studies
Electronic searches
The following electronic databases, trial registers and web sites were searched up until June 2009: The Menstrual Disorders and Subfertility Group (MDSG) Specialised Register of Controlled Trials (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), the Cochrane Central Register of Controlled Trials (Appendix 4), and PSYCINFO (Appendix 5).
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the searching chapter of The Cochrane Handbook of Systematic Reviews of Interventions. The EMBASE search was combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (http://www.sign.ac.uk/methodology/filters.html#random)
Other electronic sources of trials were included: Trial registers for ongoing and registered trials ‐ 'Current Controlled Trials' (http://www.controlled‐trials.com/), 'ClinicalTrials.gov' a service of the US national Institutes of Health (http://clinicaltrials.gov/ct2/home) and 'The World Health Organisation International Trials Registry Platform search portal' (http://www.who.int/trialsearch/Default.aspx) Citation indexes (http://scientific.thomson.com/products/sci/)
Conference abstracts in the ISI Web of Knowledge (http://isiwebofknowledge.com/) LILACS database, as a source of trials from the Portuguese and Spanish speaking world (http://bases.bireme.br/cgibin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F) ClinicalStudyResults for clinical trial results of marketed pharmaceuticals (http://www.clinicalstudyresults.org/) PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) , the random control filter for PubMed will be taken from the searching chapter of The Cochrane Handbook of Systematic.Reviews of Interventions OpenSIGLE database(http://opensigle.inist.fr/) and Google for grey literature
Searching other resources
The reference lists of articles retrieved by the search were hand searched and personal contact was made with experts in the field and with the manufacturers of growth hormone to obtain any additional relevant data. Any relevant journals and conference abstracts that are not covered in the MDSG register was hand‐searched in liaison with the Trial Search Coordinator.
Data collection and analysis
Data collection and analysis was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Selection of studies
One review author scanned retrieved searches for relevant titles and abstracts . The full text of all potentially eligible studies were retrieved. Two review authors independently examined the full text articles for compliance with the inclusion criteria and elected studies eligible for inclusion in the review. Authors corresponded with study investigators to clarify study eligibility (for example, with respect to participant eligibility criteria and allocation method). Disagreements as to study eligibility was resolved by discussion with a third author (AW).
Data extraction and management
Data was extracted from eligible studies using a data extraction form designed and pilot‐tested by the authors. Where studies have multiple publications, the main trial report was used as the reference and additional details supplemented from secondary papers. Review authors corresponded with study investigators in order to resolve data queries.Two review authors (one a methodologist and one a topic area specialist) independently extracted the data and any disagreement between these reviewer authors was resolved by a third review author (AW).
Assessment of risk of bias in included studies
The included studies were assessed for risk of bias using the Cochrane risk of bias assessment tool to assess: sequence generation; allocation concealment; blinding of participants, providers and outcome assessors; completeness of outcome data; selective outcome reporting; and other potential sources of bias. Two authors assessed these six domains, with any disagreements resolved by discussion with a third author (AW). The conclusions are presented in the Risk of Bias table and incorporated into the interpretation of review findings by means of sensitivity analyses (see below). Where identified studies failed to report the primary outcomes of live birth, but did report secondary outcomes such as clinical pregnancy, informal assessment was undertaken as to whether those reporting the primary outcomes have typical values of the secondary outcomes.
Measures of treatment effect
For dichotomous data the numbers of events in the control and intervention groups of each study was used to calculate Peto odds ratios. For continuous data standard mean differences between treatment groups was calculated if all studies report exactly the same outcomes. If similar outcomes are reported on different scales the standardised mean difference was calculated. Ordinal data was treated as continuous data. 95% confidence intervals were presented for all outcomes.
Unit of analysis issues
The primary analysis was per woman randomised. Multiple live births (e.g. twins or triplets) will be counted as one live birth event.
Dealing with missing data
The data was analysed on an intention‐to‐treat basis as far as possible and attempts were made to obtain missing data from the original investigators. Where these are unobtainable, imputation of individual values was undertaken for the primary outcomes only. Live births were assumed not to have occurred in participants with unreported outcomes. When studies reported sufficient detail to calculate mean differences but no information on associated standard deviation (SD), the outcome will be assumed to have standard deviation equal to the highest SD from other studies within the same analysis. For other outcomes, only the available data was analysed. Any imputation undertaken was subjected to sensitivity analysis (see below).
Assessment of heterogeneity
The authors considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a meaningful summary. Statistical heterogeneity was assessed by the measure of the I2. An I2 measurement greater than 50% indicates substantial heterogeneity (Higgins 2008) and where present was addressed through sensitivity and/or subgroup analysis.
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data.
Data synthesis
The data from primary studies was combined using fixed effect models in the following comparisons:
1. Routine use of adjuvant growth hormone in IVF protocols.
2. Non‐routine use of adjuvant growth hormone in IVF protocols in poor responders as defined by the study.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses was conducted in the second comparison to determine the evidence within the following sub‐groups: 1. Sub‐optimal response following controlled ovarian stimulation.
2. Poor ovarian reserve as demonstrated by abnormal ovarian reserve tests.
Sensitivity analysis
Sensitivity analyses were conducted for the primary outcomes to determine whether the conclusions are robust to arbitrary decisions made regarding the eligibility and analysis. These analyses considered whether conclusions would have differed if: 1. Eligibility were restricted to studies without high risk of bias; 2. Studies with outlying results had been excluded; 3. Alternative imputation strategies had been adopted; 4. A random effects model had been adopted.
Results
Description of studies
Results of the search
Twenty‐three randomised controlled trials were identified from the search strategy, ten of which were included in the meta‐analysis.
Included studies
Ten trials were included in the meta‐analysis. These are presented as 13 sets of data (Bergh 1994; Dor 1995; Hazout 2003; Hazout 2003 4 IU; Hazout 2003 8 IU; Kueuk 2008;Owen 1991; Suikkari 1996 ;Suikkari 1996 12 IU; Suikkari 1996 4 IU; Tapanainen 1992; Tesarik 2005;Younis 1992; Zhuang 1994). Further descriptive details about the included studies are provided in Characteristics of included studies table. All included trails were published reports (full papers or conference abstracts).
Routine use of growth hormone as an adjuvant in IVF protocols
Two trials concerned the routine use of growth hormone as an adjuvant in IVF protocols (Tapanainen 1992; Younis 1992).
Non‐routine use of growth hormone as an adjuvant in IVF protocols in women considered poor responders
The remaining eight trials considered the non‐routine use of adjuvant growth hormone in IVF protocols for subfertile couples subgrouped as:‐
1. Poor responders as described by the study (Bergh 1994; Dor 1995; Kueuk 2008; Owen 1991; Suikkari 1996; Hazout 2003; Tesarik 2005; Zhuang 1994).
2. Poor responders because of previous sub‐optimal response following controlled ovarian stimulation (Bergh 1994; Dor 1995; Kueuk 2008; Owen 1991; Suikkari 1996).
3. Poor responders because of poor ovarian reserve as demonstrated by abnormal ovarian reserve tests ‐ no trials identified.
Participants
Ten trials with a total of 440 subfertile couples were included in the meta‐analysis. The number of couples included in each trial ranged from 14 (Dor 1995) to 61 (Kueuk 2008). All studies detailed the age ranges and included women aged 30 to 40 years old with exception of Tesarik 2005 who included women aged forty years or older. Exclusion criteria were not stated in Dor 1995, Hazout 2003, Owen 1991, Suikkari 1996, Tapanainen 1992 and Zhuang 1994. The remaining trials based their exclusion criteria on serum FSH concentrations (Kueuk 2008; Tesarik 2005), obesity (Bergh 1994), ovarian pathology (Bergh 1994), endometriosis (Bergh 1994), severe intercurrent illness (Bergh 1994) and unsatisfactory sperm quality (Tesarik 2005).
Interventions
There was no consistency as to the dose or timing of growth hormone administration (Please see Characteristics of included studies table). The dose of growth hormone ranged from 8IU (Tesarik 2005) to 24IU (Owen 1991; Tapanainen 1992). Both Hazout 2003 and Suikkari 1996 conducted a multi‐arm trail comparing two different doses of growth hormone to a control arm. For the purposes of comparison the separate arms were allocated two different study IDs. The timing of growth hormone administration varied between studies from daily administration to alternate days.
Outcomes
Primary outcome measure
Live birth rates were reported by seven of the included trials (Dor 1995; Owen 1991; Tapanainen 1992; Tesarik 2005; Suikkari 1996; Younis 1992; Zhuang 1994).
Secondary outcomes measures
Pregnancy rates were reported by eight of the included trials (Bergh 1994; Hazout 2003; Kueuk 2008; Owen 1991; Suikkari 1996; Tesarik 2005; Younis 1992; Zhuang 1994) . Two trials reported the number of oocytes retrieved per woman (Bergh 1994; Younis 1992). Three trials reported the embryo transfer rate (Bergh 1994; Younis 1992; Suikkari 1996). Two trials reported the mean number of ampoules of gonadotrophin used per woman randomised (Tapanainen 1992; Younis 1992). Adverse effects were reported by four of the trials (Owen 1991; Suikkari 1996; Tapanainen 1992; Younis 1992).
Excluded studies
Thirteen trials were excluded outlined in the Characteristics of excluded studies table. Six trials were excluded because the participants did not undergo IVF (Blumenfeld 1994; Busacca 1996; Homburg 1990b; Homburg 1995; Jacobs 1995; Landolfi 1994;Tulandi 1993). A further trial were excluded because growth hormone was not the intervention (Howles 1999; Schoolcraft 1997). Three trials were excluded because the study was not truly randomised (Homburg 1990; Owen 1991b; Rinehart 1999).
Risk of bias in included studies
Please refer to Characteristics of included studies table, Figure 1 and Figure 2.
1.
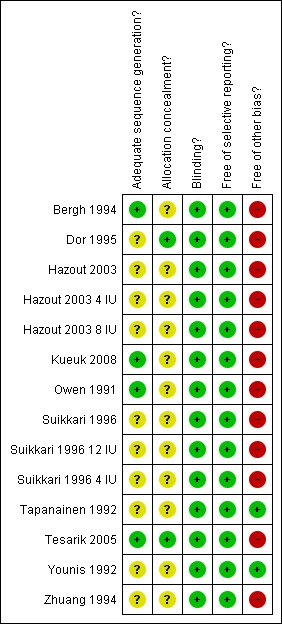
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
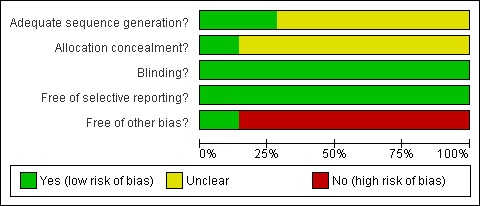
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
The method of randomisation was clearly stated in five trials (Kueuk 2008; Tapanainen 1992, Tesarik 2005, Suikkari 1996 & Younis 1992). The method of randomisation was unclear in the remaining trails. Allocation concealment was unclear in all the included trails, except Kueuk 2008, Tapanainen 1992, Tesarik 2005 and Younis 1992.
Blinding
One trial (Zhuang 1994) reported single blinding, seven trials were double blinded (Bergh 1994; Dor 1995; Hazout 2003; Owen 1991; Suikkari 1996; Tapanainen 1992; Tesarik 2005) and a two trials reported triple‐blinded (Kueuk 2008; Younis 1992).
Incomplete outcome data
Two women were lost to follow up in the Bergh 1994 study and four women were lost to follow up in the Suikkari 1996 study. The remaining trials reported no losses. Several study authors replied to requests for additional information including Bergh 1994, Dor 1995, Tapanainen 1992, Younis 1992 and Zhuang 1994.
Selective reporting
No selective reporting was identified.
Other potential sources of bias
A number of trials received a free supply of growth hormone from the manufacture including Owen 1991, Tapanainen 1992 and Younis 1992. One trial reported a withdrawal or cycle cancellation rate greater than 10% of participants (Suikkari 1996). Owen 1991 did not describe the nature of the placebo.
Effects of interventions
The effects of adjuvant growth hormone in IVF protocols are reported in the following populations:‐
1. Women who are not considered poor responders.
2. Women who are considered poor responders as defined by the study.
3. Women who are considered poor responders because of a previous sub‐optimal response following controlled ovarian stimulation.
The use of adjuvant growth hormone in IVF protocols in women who are not considered poor responders.
Main outcome measure
Live birth rate per woman randomised
Two trials reported the live birth rate per woman randomised (Tapanainen 1992; Younis 1992). Meta‐analysis demonstrated no difference in the use growth hormone adjuvant in IVF protocols when compared to standard IVF protocols OR 1.32, 95% CI 0.40 to 4.43 ; 80 participants , 2 trials Analysis 1.1 (Figure 3).
1.1. Analysis.
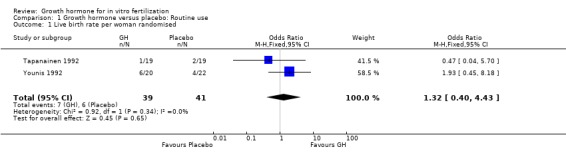
Comparison 1 Growth hormone versus placebo: Routine use, Outcome 1 Live birth rate per woman randomised.
3.
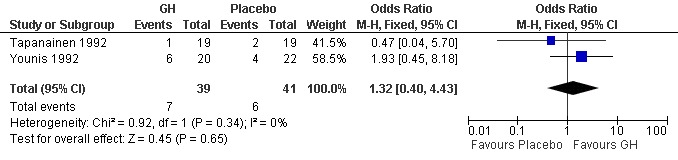
Forest plot of comparison: 1 Growth hormone versus placebo: Routine use, outcome: 1.1 Live birth rate per woman randomised.
Additional outcomes measures
Pregnancy rate per woman randomised
One trial reported the pregnancy rate per woman randomised (Younis 1992). Analysis demonstrated no difference in the use growth hormone adjuvant in IVF protocols when compared to standard IVF protocols OR 1.78, 95% CI 0.49 to 6.50 ; 42 participants , 1 trials , Analysis 1.2 (Figure 4).
1.2. Analysis.
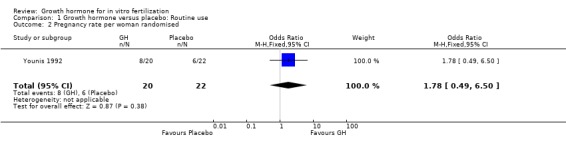
Comparison 1 Growth hormone versus placebo: Routine use, Outcome 2 Pregnancy rate per woman randomised.
4.
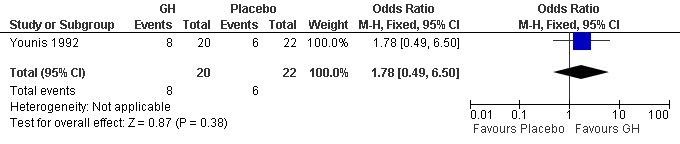
Forest plot of comparison: 1 Growth hormone versus placebo: Routine use, outcome: 1.2 Pregnancy rate per woman randomised.
Oocytes retrieved per woman randomised.
One trial reported the live birth rate per woman randomised (Younis 1992). Analysis demonstrated no difference in the use growth hormone adjuvant in IVF protocols when compared to standard IVF protocols OR 2.86, 95% CI 0.11 to 74.31 ; 42 participants , 1 trial Analysis 1.3 (Figure 5).
1.3. Analysis.
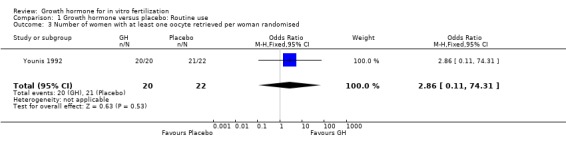
Comparison 1 Growth hormone versus placebo: Routine use, Outcome 3 Number of women with at least one oocyte retrieved per woman randomised.
5.

Forest plot of comparison: 1 Growth hormone versus placebo: Routine use, outcome: 1.3 Number of women with at least one oocyte retrieved per woman randomised.
Embryo transfer per woman randomised
One trial reported the number of embryos transferred per woman randomised (Younis 1992). Analysis demonstrated no difference in the use growth hormone adjuvant in IVF protocols when compared to standard IVF protocols OR 7.36, 95% CI 0.36 to 151.91 ; 42 participants , 1 trial Analysis 1.4 (Figure 6).
1.4. Analysis.
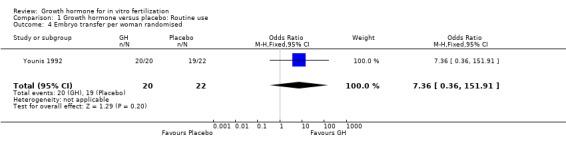
Comparison 1 Growth hormone versus placebo: Routine use, Outcome 4 Embryo transfer per woman randomised.
6.

Forest plot of comparison: 1 Growth hormone versus placebo: Routine use, outcome: 1.4 Embryo transfer per woman randomised.
Mean ampoules of gonadotrophin used.
Two trials (Tapanainen 1992; Younis 1992) reported the mean number of ampoules of gonadotrophin used per woman randomised. Meta‐analysis demonstrated no difference in the use growth hormone adjuvant in IVF protocols when compared to standard IVF protocols OR 0.18, 95% CI ‐1.53 to 1.87 ; 80 participants , 2 trials Analysis 1.5 (Figure 7).
1.5. Analysis.
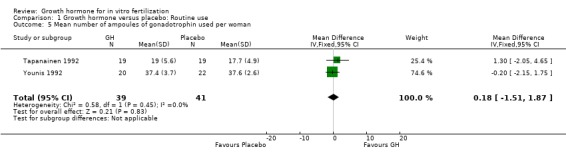
Comparison 1 Growth hormone versus placebo: Routine use, Outcome 5 Mean number of ampoules of gonadotrophin used per woman.
7.
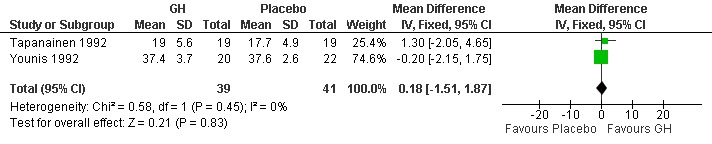
Forest plot of comparison: 1 Growth hormone versus placebo: Routine use, outcome: 1.5 Mean number of ampoules of gonadotrophin used per woman.
Adverse events
Two trials reported the occurrence of adverse events (Tapanainen 1992; Younis 1992). Meta‐analysis demonstrated no difference in the use growth hormone adjuvant in IVF protocols when compared to standard IVF protocols OR 0.62, 95% CI 0.18 to 2.15 ; 80 participants , 2 trials Analysis 1.6 (Figure 8).
1.6. Analysis.
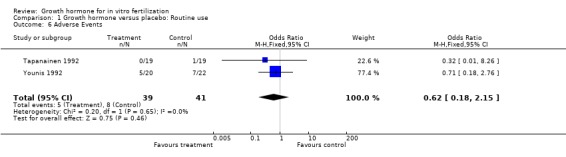
Comparison 1 Growth hormone versus placebo: Routine use, Outcome 6 Adverse Events.
8.
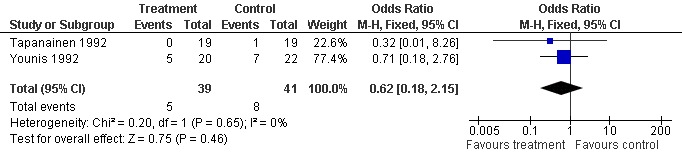
Forest plot of comparison: 1 Growth hormone versus placebo: Routine use, outcome: 1.6 Adverse Events.
The use of adjuvant growth hormone in IVF protocols in women who are considered poor responders as defined by the included study.
Main outcome measure
Live birth rate per woman randomised
Four trials reported the live birth rate per woman randomised (Owen 1991; Suikkari 1996 4 IU; Tesarik 2005;Zhuang 1994). Meta‐analysis demonstrated a statistically significant difference favouring the use growth hormone adjuvant in IVF protocols when compared to IVF protocols in women with a poor prognosis as defined by the included study OR 5.39, 95% CI 1.89 to 15.35 ; 165 participants , 4 trials , Analysis 2.1 (Figure 9).
2.1. Analysis.
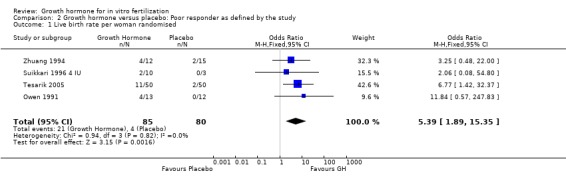
Comparison 2 Growth hormone versus placebo: Poor responder as defined by the study, Outcome 1 Live birth rate per woman randomised.
9.
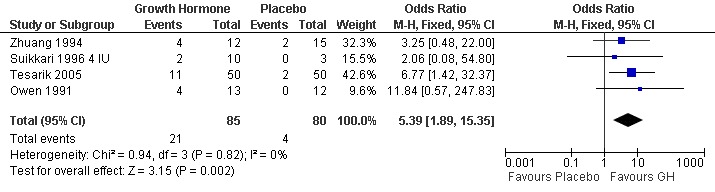
Forest plot of comparison: 2 Growth hormone versus placebo: Poor responder as defined by the study, outcome: 2.1 Live birth rate per woman randomised.
Additional outcomes measures
Pregnancy rate per woman randomised
Seven of the trials reported the pregnancy birth rate per woman randomised (Bergh 1994; Hazout 2003 4 IU; Hazout 2003 8 IU; Kueuk 2008; Owen 1991; Suikkari 1996 4 IU; Tesarik 2005; Zhuang 1994). Meta‐analysis demonstrated a statistically significant difference favouring the use growth hormone adjuvant in IVF protocols when compared to IVF protocols in women with a poor prognosis as defined by the included study OR 3.28, 95% CI 1.74 to 6.20 ; 279 participants , 7 trials , Analysis 2.2 (Figure 10).
2.2. Analysis.
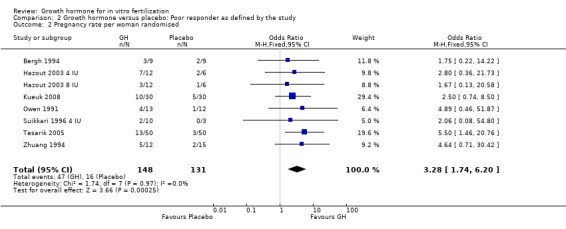
Comparison 2 Growth hormone versus placebo: Poor responder as defined by the study, Outcome 2 Pregnancy rate per woman randomised.
10.
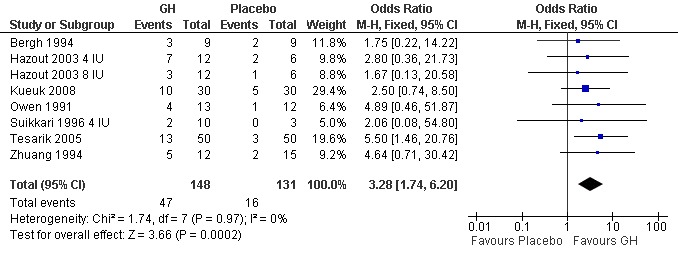
Forest plot of comparison: 2 Growth hormone versus placebo: Poor responder as defined by the study, outcome: 2.2 Pregnancy rate per woman randomised.
Oocytes retrieved per woman randomised
One trial reported the oocytes retrieved per woman randomised (Bergh 1994). Analysis could not be performed because the same number of events occurred in each group, and the groups involved the same number of couples Analysis 3.3 (Figure 11).
3.3. Analysis.
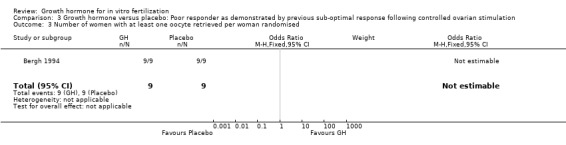
Comparison 3 Growth hormone versus placebo: Poor responder as demonstrated by previous sub‐optimal response following controlled ovarian stimulation, Outcome 3 Number of women with at least one oocyte retrieved per woman randomised.
11.

Forest plot of comparison: 3 Growth hormone versus placebo: Poor responder as demonstrated by previous sub‐optimal response following controlled ovarian stimulation, outcome: 3.3 Number of women with at least one oocyte retrieved per woman randomised.
Embryo transfer per woman randomised
Two trials reported the number of embryos transferred per woman randomised (Bergh 1994; Suikkari 1996 4 IU; Suikkari 1996 12 IU). Meta‐analysis demonstrated no difference in the use growth hormone adjuvant in IVF protocols when compared to IVF protocols in women with a sub‐optimal response following controlled ovarian stimulation OR 2.01, 95% CI 0.38 to 10.78 ; 40 participants , 2 trials , Analysis 3.4 (Figure 12).
3.4. Analysis.
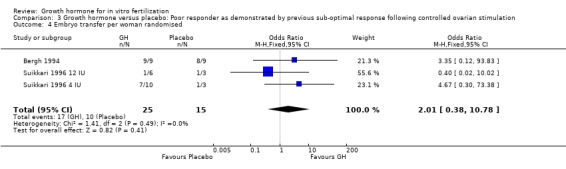
Comparison 3 Growth hormone versus placebo: Poor responder as demonstrated by previous sub‐optimal response following controlled ovarian stimulation, Outcome 4 Embryo transfer per woman randomised.
12.
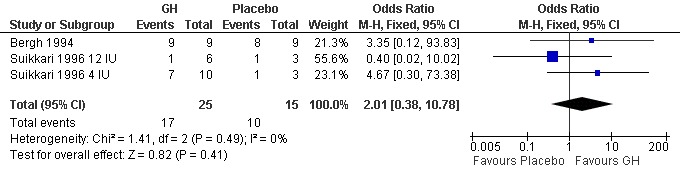
Forest plot of comparison: 3 Growth hormone versus placebo: Poor responder as demonstrated by previous sub‐optimal response following controlled ovarian stimulation, outcome: 3.4 Embryo transfer per woman randomised.
Adverse events
One trial reported the oocytes retrieved per woman randomised (Owen 1991). Analysis demonstrated no difference in the use growth hormone adjuvant in IVF protocols when compared to IVF protocols in women with a sub‐optimal response following controlled ovarian stimulation OR 2.00, 95% CI 0.16 to 25.40 ; 116 participants , 1 trial , Analysis 3.5 (Figure 13).
3.5. Analysis.
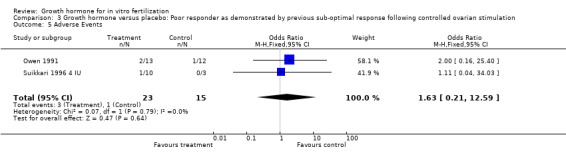
Comparison 3 Growth hormone versus placebo: Poor responder as demonstrated by previous sub‐optimal response following controlled ovarian stimulation, Outcome 5 Adverse Events.
13.
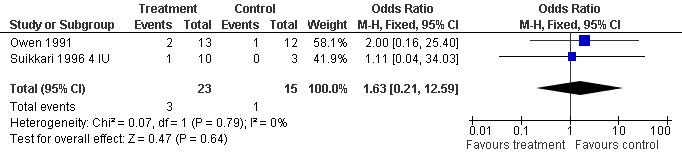
Forest plot of comparison: 3 Growth hormone versus placebo: Poor responder as demonstrated by previous sub‐optimal response following controlled ovarian stimulation, outcome: 3.5 Adverse Events.
Sensitivity Analysis
One trial reported a withdrawal or cycle cancellation rate greater than 10% of participants (Suikkari 1996). A sensitivity analysis was performed to detect whether the inclusion of this randomised controlled trials affected the results. There was no difference in results when the meta‐analysis was re‐calculated.
The use of adjuvant growth hormone in IVF protocols in women who are considered poor responders because of a previous sub‐optimal response following controlled ovarian stimulation
Main outcome measure
Live birth rate per woman randomised
Two trials reported the live birth rate per woman randomised (Owen 1991; Suikkari 1996 4 IU). Meta‐analysis demonstrated no difference in the use growth hormone adjuvant in IVF protocols when compared to IVF protocols in women with a sub‐optimal response following controlled ovarian stimulation OR 5.81, 95% CI 0.67 to 50.39 ; 38 participants , 2 trials , Analysis 3.1 (Figure 14).
3.1. Analysis.
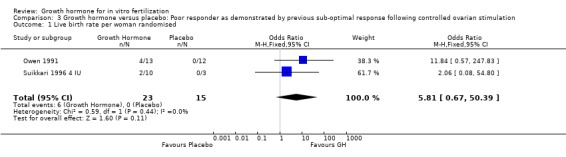
Comparison 3 Growth hormone versus placebo: Poor responder as demonstrated by previous sub‐optimal response following controlled ovarian stimulation, Outcome 1 Live birth rate per woman randomised.
14.
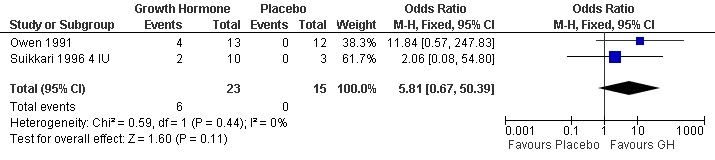
Forest plot of comparison: 3 Growth hormone versus placebo: Poor responder as demonstrated by previous sub‐optimal response following controlled ovarian stimulation, outcome: 3.1 Live birth rate per woman randomised.
Additional outcomes measures
Pregnancy rate per woman randomised
Four of the trials reported the pregnancy birth rate per woman randomised (Bergh 1994; Kueuk 2008; Owen 1991; Suikkari 1996 4 IU). Meta‐analysis demonstrated a statistically significant difference favouring the use growth hormone adjuvant in IVF protocols when compared to IVF protocols in women with a sub‐optimal response following controlled ovarian stimulation OR 2.58, 95% CI 1.03 to 6.46 ; 116 participants , 4 trials , Analysis 3.2 (Figure 15).
3.2. Analysis.
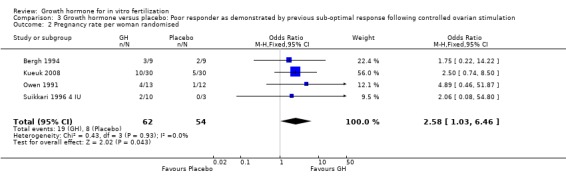
Comparison 3 Growth hormone versus placebo: Poor responder as demonstrated by previous sub‐optimal response following controlled ovarian stimulation, Outcome 2 Pregnancy rate per woman randomised.
15.
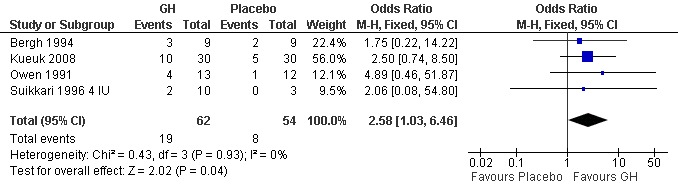
Forest plot of comparison: 3 Growth hormone versus placebo: Poor responder as demonstrated by previous sub‐optimal response following controlled ovarian stimulation, outcome: 3.2 Pregnancy rate per woman randomised.
Oocytes retrieved per woman randomised
One trial reported the oocytes retrieved per woman randomised (Bergh 1994). Analysis could not be performed because the same number of events occurred in each group, and the groups involved the same number of couples Analysis 3.3 (Figure 11).
Embryo transfer per woman randomised
Two trials reported the number of embryos transferred per woman randomised (Bergh 1994; Suikkari 1996 4 IU; Suikkari 1996 12 IU). Meta‐analysis demonstrated no difference in the use growth hormone adjuvant in IVF protocols when compared to IVF protocols in women with a sub‐optimal response following controlled ovarian stimulation OR 2.01, 95% CI 0.38 to 10.78 ; 40 participants , 2 trials , Analysis 3.4 (Figure 12).
Adverse events
One trial reported the oocytes retrieved per woman randomised (Owen 1991). Analysis demonstrated no difference in the use growth hormone adjuvant in IVF protocols when compared to IVF protocols in women with a sub‐optimal response following controlled ovarian stimulation OR 2.00, 95% CI 0.16 to 25.40 ; 116 participants , 1 trial , Analysis 3.5 (Figure 13).
Sensitivity Analysis
One trial reported a withdrawal or cycle cancellation rate greater than 10% of participants (Suikkari 1996). A sensitivity analysis was performed to detect whether the inclusion of this randomised controlled trials affected the results. There was no difference in results when the meta‐analysis was re‐calculated.
Discussion
Summary of main results
This review was undertaken to establish the role of growth hormone adjuvant therapy for IVF in improving IVF outcomes particularly in those women who are considered poor responders. Results of this meta‐analysis demonstrated no difference in IVF outcome measures and adverse events in the routine use of growth hormone adjuvant therapy in IVF. However, meta‐analysis demonstrated a statistically significant difference in both live birth rates and pregnancy rates favouring the use of growth hormone adjuvant therapy in IVF in women who are considered poor responders without increasing adverse events (Analysis 2.1, Analysis 2.2, Analysis 3.5). Although these results have to be interpreted with caution, the trials included in the meta‐analysis were few and of small sample size.
Defining which sub‐group or sub‐groups of poor responder benefited the most from growth hormone adjuvant therapy in IVF proved challenging due to the diverse definitions of poor responder used by the included studies. Sub‐group analysis demonstrated a statistically significant difference in pregnancy rates favouring the use of growth hormone adjuvant in IVF in women who are considered poor responders because of previous sub‐optimal response following controlled ovarian stimulation without increasing adverse events. Although these results have to be interpreted with caution, the trials included in the meta‐analysis were few and of small sample size. (Analysis 3.2, Analysis 3.5). However, meta‐analysis demonstrated no difference in the other IVF outcome measures, including live birth rate, in the use of growth hormone adjuvant in IVF in these women (Analysis 3.1).
Overall completeness and applicability of evidence
Meta‐analysis demonstrated a statistically significant difference in both live birth rates and pregnancy rates favouring the use of growth hormone adjuvant in IVF in women who are considered poor responders without increasing adverse events (Analysis 2.1, Analysis 2.2, Analysis 3.5). Sub‐group analysis demonstrated a statistically significant difference in pregnancy rates favouring the use of growth hormone adjuvant in IVF in women who are considered poor responders because of previous sub‐optimal response following controlled ovarian stimulation without increasing adverse events (Analysis 3.2, Analysis 3.5). The width of the confidence interval should be taken into account when considering the results. The wide confidence interval emphasises the lack of available evidence, the included trials were few in number and of small sample size for the primary outcome, live birth rates, in the sub‐group analysis.
Adverse effects were also considered as a secondary outcome. Frequency of reporting of adverse effects varied between the trials and different adverse effects were recorded. In general adverse effects were not well documented making the meta‐analysis result fairly unreliable as emphasised by the wide confidence intervals. For those that were documented, growth hormone adjuvant in IVF protocols did not significantly reduce the incidence of any of the adverse effects in either group.
The causative factors for poor response to controlled controlled ovarian hyperstimulation are not well described in the literature. Consequently the definitions of a 'poor responder' are varied ranging from age to poor responders to gonadotrophin stimulation on previous IVF cycles. Therefore the inclusion criteria of the included trials varied greatly. Therefore we have been unable to identify the particular sub‐group of poor responders who would benefit the most from growth hormone augmentation in IVF protocols in terms of live birth rates.
Quality of the evidence
Of the ten randomised controlled trials included in the review differences in participant number, cause of subfertility, treatment protocol and outcomes measured all varied considerably between the trials. There was no uniformity of dose and timing of the intervention. A large scale trial with a standardised treatment protocol and intervention protocol is required (Please refer to Implications for research section).
There was a lack of large high quality trials comparing growth hormone to placebo in ovarian stimulation protocols in IVF cycles. If a new large randomised controlled trial was performed the results of this review could be significantly different.
Potential biases in the review process
Critical to the limitation of bias in these included randomised controlled trials are the randomisation method and allocation concealment strategy deployed, both of which provide similar comparison groups achieving a balance of both known and unknown factors that may influence the outcome. Six of the ten included trials did not state explicitly the method of randomisation used. Failure to report how women were randomised does not allow us to evaluate the adequacy of the method deployed. Furthermore, seven of the ten included randomised controlled trials did not state a method of allocation concealment. This could undermine further the quality of the included randomised controlled trial. With poor methods of concealing the allocation, knowledge of the treatment codes may be gained in advance, increasing the likelihood of selection bias (Li 2005).
One trial reported a withdrawal or cycle cancellation rate greater than 10% of participants (Suikkari 1996). A sensitivity analysis was performed to detect whether the inclusion of this randomised controlled trial affected the results. There was no difference in results when the meta‐analysis was re‐calculated.
Owen 1991 did not describe the nature of the placebo which could have lead to bias if the placebo had an action mechanism similar to growth hormone
Agreements and disagreements with other studies or reviews
Currently no national or international guidelines recommend the routine use of growth hormone augmentation in IVF protocols. However a recent systematic review and meta‐analysis concerning the evaluation of strategies to improve the pregnancy rates in poor responders undergoing IVF concluded that there was some evidence to suggest the addition of growth hormone could improve live birth rates but further research was required (Kyrou 2009). Kyrou and colleagues analysis and conclusions broadly agree with our own.
Other studies have reported mixed results. Rinehart and colleagues reported that growth hormone did not significantly increase the pregnancy rates for women who were defined as 'poor responders' to gonadotrophin stimulation on previous IVF cycles. The study, which was non‐randomised in design, and defined a poor responder as one whose follicles did not reach 18mm in diameter or an E2 of less than 500 pg/ml (Rinehart 1999). In a cross‐over trial Blumenfeld 1996 (Blumenfeld 1996) examined the role of growth hormone augmentation in poor responders to gonadotrophin stimulation on previous IVF cycles. Interestingly the study concluded that the addition of growth hormone was only beneficial in terms of pregnancy and live birth rates in women who were not 'endocrinologically normal' as illustrated by being identified as clonidine positive.
Other studies have evaluated the potential for the use of growth hormone releasing factor. Growth hormone releasing factor may also have a role in ovulation induction for IVF. Pituitary growth hormone secretion is controlled by growth hormone releasing factor which may also have a direct effect on the ovary. A pilot study demonstrated that growth hormone releasing factor was associated with improvement in ovarian response and resulted in slight increases in recruited follicles and retrieved oocytes (Hughes 1994). Growth hormone releasing factor seems to have a similar effect as growth hormone on ovarian response (Howles 1999). Howles and colleagues performed a randomised controlled trial and reported that growth hormone releasing factor did not significantly increase the pregnancy or live birth rates for women who demonstrate as poor responders to gonadotrophin stimulation on previous IVF cycles.
Authors' conclusions
Implications for practice.
In women who are not considered poor responders undergoing in IVF there is no evidence from randomised controlled trials to support the use of growth hormone. In women who are considered poor responders the use of growth hormone has been shown to significantly improve live birth and pregnancy rates. Although the exact sub‐group of poor responders who would benefit from growth hormone augmentation needs to be identified. The result needs to be interpreted with caution, the included trials were few in number and small with significant clinical heterogeneity.
Implications for research.
With regards to women who are known poor responders to IVF, a multi‐centre randomised double blinded trial is warranted to investigate the effect of growth hormone augmentation. Key elements of design should include power calculation to ensure the minimum number of participants needed for a significant result are included, the standardisation of controlled controlled ovarian hyperstimulation protocols, dose of growth hormone and the definition of a poor responder ‐ <4 oocytes retrieved in a previous IVF attempt might be appropriate. The primary outcome of live birth rate should be measured. Only by considering such outcomes can this therapy be truly tested. Also, adverse effects, for example OHSS and miscarriage, should be routinely reported. Given the high hormone cost of treatment, one component of this study should also be an economic evaluation.
What's new
| Date | Event | Description |
|---|---|---|
| 20 September 2010 | Amended | Contact details updated. |
History
Protocol first published: Issue 1, 1995 Review first published: Issue 1, 1995
| Date | Event | Description |
|---|---|---|
| 24 August 2009 | New citation required but conclusions have not changed | Authors changed |
| 11 August 2009 | New citation required but conclusions have not changed | New authors added |
| 14 June 2009 | New search has been performed | Since the last published review (1995 & 2003), the authorship of the review has changed. New authors involved in updating the review in 2009 included G Ahmad, J Brown, JMN Duffy, L Nardo, I Salim and AJ Watson. New randomised controlled trials were included in the review, resulting from repeating the search strategy In June 2009. Subgroup analysis of poor responders was performed in the 2009 update, the first subgroup defined as poor responders as demonstrated by sub‐optimal response following controlled ovarian stimulation and the second subgroup defined as poor ovarian performance as demonstrated by abnormal ovarian reserve tests. |
| 28 April 2008 | Amended | Converted to new review format. |
| 28 May 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the members of the Cochrane Menstrual Disorders and Subfertility Review Group based in Auckland, New Zealand, who assisted with this review.
Appendices
Appendix 1. MDSG search terms
Search string for KH291 MDSG database 28.06.09
Keywords CONTAINS "growth hormone" or "growth hormone derivative" or "human growth hormone" or "growth hormone releasing factor" or "grf" or Title CONTAINS "growth hormone" or "growth hormone derivative" or "human growth hormone" or "growth hormone releasing factor" or "grf"
AND
Keywords CONTAINS "IVF" or "in vitro fertilization" or "IVF" or "ICSI" or "intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or Title CONTAINS "IVF" or "in vitro fertilization" or "IVF" or "ICSI" or"intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization"
Appendix 2. MEDLINE search strategy
Database: Ovid MEDLINE(R) <1950 to June Week 3 2009> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 growth hormone/ or human growth hormone/ (47027) 2 somatotrop$.tw. (5778) 3 (somatrop$ or norditropin).tw. (184) 4 (growth adj5 hormone$).tw. (48524) 5 or/1‐4 (64412) 6 fertilization in vitro/ or sperm injections, intracytoplasmic/ (22271) 7 IVF.tw. (11335) 8 (in Vitro adj5 fertili$).tw. (14828) 9 icsi.tw. (3589) 10 (intracytoplas$ adj5 sperm).tw. (3510) 11 exp Ovulation Induction/ (8201) 12 ((ovar$ or ovulat$) adj5 (induct$ or stimulat$)).tw. (9654) 13 or/6‐12 (37952) 14 exp growth hormone‐releasing hormone/ or exp sermorelin/ (4583) 15 (growth hormone adj5 releasing factor).tw. (1341) 16 grf.tw. (1855) 17 or/14‐16 (5630) 18 or/5,17 (65464) 19 18 and 13 (460) 20 randomized controlled trial.pt. (273632) 21 controlled clinical trial.pt. (79523) 22 randomized.ab. (183258) 23 placebo.tw. (116263) 24 clinical trials as topic.sh. (144111) 25 randomly.ab. (132970) 26 trial.ti. (79814) 27 (crossover or cross‐over or cross over).tw. (43128) 28 or/20‐27 (648343) 29 (animals not (humans and animals)).sh. (3296848) 30 28 not 29 (600179) 31 30 and 19 (71)
Appendix 3. EMBASE search strategy
Database: EMBASE <1980 to 2009 Week 26> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 growth hormone/ or growth hormone derivative/ or human growth hormone/ (37552) 2 somatotrop$.tw. (3622) 3 (somatrop$ or norditropin).tw. (653) 4 (growth adj5 hormone$).tw. (39350) 5 or/1‐4 (52850) 6 fertilization in vitro/ or intracytoplasmic sperm injection/ (23933) 7 IVF.tw. (11213) 8 (in Vitro adj5 fertili$).tw. (12921) 9 icsi.tw. (3793) 10 (intracytoplas$ adj5 sperm).tw. (3461) 11 ovary hyperstimulation/ or ovulation induction/ (9384) 12 ((ovar$ or ovulat$) adj5 (induct$ or stimulat$)).tw. (8632) 13 or/6‐12 (35212) 14 5 and 13 (468) 15 exp growth hormone releasing factor/ or exp "growth hormone releasing factor[1‐29]"/ (4982) 16 (growth hormone adj5 releasing factor).tw. (1135) 17 grf.tw. (1494) 18 or/15‐17 (5770) 19 or/5,18 (53914) 20 19 and 13 (479) 21 Clinical Trial/ (545660) 22 Randomized Controlled Trial/ (170304) 23 exp randomization/ (26900) 24 Single Blind Procedure/ (8278) 25 Double Blind Procedure/ (72902) 26 Crossover Procedure/ (21458) 27 Placebo/ (128084) 28 Randomi?ed controlled trial$.tw. (33843) 29 randomised controlled trials.tw. (2814) 30 random allocation.tw. (641) 31 randomly allocated.tw. (10334) 32 allocated randomly.tw. (1359) 33 (allocated adj2 random).tw. (562) 34 Single blind$.tw. (7573) 35 Double blind$.tw. (85680) 36 ((treble or triple) adj blind$).tw. (140) 37 placebo$.tw. (111457) 38 prospective study/ (83224) 39 or/21‐38 (716803) 40 case study/ (6169) 41 case report.tw. (120958) 42 abstract report/ or letter/ (502683) 43 or/40‐42 (627435) 44 39 not 43 (691822) 45 44 and 20 (92)
Appendix 4. CENTRAL search Strategy
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <2nd Quarter 2009> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 growth hormone/ or human growth hormone/ (2525) 2 somatotrop$.tw. (157) 3 (somatrop$ or norditropin).tw. (43) 4 (growth adj5 hormone$).tw. (3238) 5 or/1‐4 (3704) 6 fertilization in vitro/ or sperm injections, intracytoplasmic/ (1223) 7 ivf.tw. (1610) 8 (in Vitro adj5 fertili$).tw. (1203) 9 icsi.tw. (547) 10 (intracytoplas$ adj5 sperm).tw. (339) 11 exp Ovulation Induction/ (717) 12 ((ovar$ or ovulat$) adj5 (induct$ or stimulat$)).tw. (1076) 13 or/6‐12 (3192) 14 exp growth hormone‐releasing hormone/ or exp sermorelin/ (300) 15 (growth hormone adj5 releasing factor).tw. (43) 16 grf.tw. (71) 17 or/14‐16 (344) 18 or/5,17 (3736) 19 18 and 13 (64) 20 limit 19 to yr="2007 ‐Current" (2) 21 from 20 keep 1‐2 (2)
Appendix 5. psycINFO search strategy
Database: PsycINFO <1806 to June Week 1 2009> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 growth hormone/ or human growth hormone/ (969) 2 somatotrop$.tw. (151) 3 (somatrop$ or norditropin).tw. (5) 4 (growth adj5 hormone$).tw. (1777) 5 or/1‐4 (1922) 6 fertilization in vitro/ or sperm injections, intracytoplasmic/ (0) 7 ivf.tw. (239) 8 (in Vitro adj5 fertili$).tw. (353) 9 icsi.tw. (24) 10 (intracytoplas$ adj5 sperm).tw. (16) 11 exp Ovulation Induction/ (0) 12 ((ovar$ or ovulat$) adj5 (induct$ or stimulat$)).tw. (177) 13 or/6‐12 (581) 14 exp growth hormone‐releasing hormone/ or exp sermorelin/ (0) 15 (growth hormone adj5 releasing factor).tw. (41) 16 grf.tw. (45) 17 or/14‐16 (63) 18 or/5,17 (1943) 19 18 and 13 (3) 20 from 19 keep 1‐3 (3)
Data and analyses
Comparison 1. Growth hormone versus placebo: Routine use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per woman randomised | 2 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.40, 4.43] |
| 2 Pregnancy rate per woman randomised | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.49, 6.50] |
| 3 Number of women with at least one oocyte retrieved per woman randomised | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.11, 74.31] |
| 4 Embryo transfer per woman randomised | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.36 [0.36, 151.91] |
| 5 Mean number of ampoules of gonadotrophin used per woman | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐1.51, 1.87] |
| 6 Adverse Events | 2 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.18, 2.15] |
Comparison 2. Growth hormone versus placebo: Poor responder as defined by the study.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per woman randomised | 4 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.39 [1.89, 15.35] |
| 2 Pregnancy rate per woman randomised | 8 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.28 [1.74, 6.20] |
Comparison 3. Growth hormone versus placebo: Poor responder as demonstrated by previous sub‐optimal response following controlled ovarian stimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per woman randomised | 2 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.81 [0.67, 50.39] |
| 2 Pregnancy rate per woman randomised | 4 | 116 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.58 [1.03, 6.46] |
| 3 Number of women with at least one oocyte retrieved per woman randomised | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Embryo transfer per woman randomised | 3 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.38, 10.78] |
| 5 Adverse Events | 2 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.21, 12.59] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bergh 1994.
| Methods | Randomisation: Using a computerised list women were randomised to one of four arms. Allocation Concealment: unclear. Blinding: double‐blind. Trial Design: Parallel. Analysis: power calculation was performed, no intention to treat analysis performed. Study Setting: Multicentre study ‐ three IVF programs in Sweden. Withdrawals: two women (< 10%). Cancelled cycles: one woman in placebo group (<10%). |
|
| Participants | Number of women: 18 (nine growth hormone, nine placebo). IVF previous poor responders: at least two failed cycles with < five oocytes. Regular menstrual cycle, normal FSH, LH, PRL and ovarian ultrasound. BMI less than or equal to 28, age 25 to 38 years. Normal semen quality, (WHO criteria). | |
| Interventions | Intervention: growth hormone 0.1 IU/kg daily subcutaneous. Treatment Protocol: seven days pretreatment with placebo; pre‐treatment was started after ovarian down regulation was established (achieved with BA beginning on day one or two of cycle administered intranasally six/day or in a few cases by s/c injection two per day for a total dose of 1.2mg/day. Treatment with BA continued during the pre‐treatment and stimulation periods). Ovarian stimulation was performed by hMG 225 to 300 IU/day IM and/or FSH in a dose of 75 to 300 IU/day for 10 to 25 days. Protocol, n=10 women and cycles. Dose of human chorionic gonadotropin: 10000 IU when at least one follicle was >18mm diameter and there had been seven to eight days of continued rise of serum E2. |
|
| Outcomes | Pregnancy rate, oocyte retrieval and embryo transfer. | |
| Notes | This trial involved four treatment arms (and 40 women) but only data comparing growth hormone use in conjunction with GnRHa / hMG vs standard treatment (groups I, II) were included. Groups III and IV involved growth hormone pretreatment and were excluded. The placebo used was NaCl. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation: Using a computerised list women were randomised to one of four arms |
| Allocation concealment? | Unclear risk | Not stated within the text |
| Blinding? All outcomes | Low risk | Double‐blinded |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | High risk | The study does not report on adverse effects or multiple pregnancies |
Dor 1995.
| Methods | Randomisation: stated as randomised. Allocation concealment: sealed opaque sequentially numbered, identical envelopes. Blinding: double‐blind. Trial Design: Parallel. Analysis: No power calculation or intention to treat analysis performed. Study setting: single centre, location Israel Withdrawals: none (<10%). Cancelled cycles: <10%. |
|
| Participants | Number of women: 14 (seven growth hormone, seven placebo). Inclusion Criteria: IVF previous poor responders defined as E2 < 500 pg/ml on day of human chorionic gonadotropin, < three oocytes retrieved. Normal serum FSH, LH levels. Cause of Subfertility: ovulatory disorders or tubal factor infertility. Age: 30 to 45. |
|
| Interventions | Intervention: growth hormone 18 IU SC on days two, four, six, and eight of stimulation. Treatment Protocol: Short GnRHa/FSH/hMG protocol used, SC on cycle days two, four, six, and eight. Dose of human chorionic gonadotropin: 10000 IU when serum oestradiol was >200pg/ml and at least two follicles were > 18mm in diameter. | |
| Outcomes | Pregnancy rate. | |
| Notes | Mannitol chosen as placebo because "no known ovarian effects." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated as 'randomised' no other details |
| Allocation concealment? | Low risk | Sealed opaque sequentially numbered, identical envelopes |
| Blinding? All outcomes | Low risk | Double blinded |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | High risk | No adverse effects reported |
Hazout 2003.
| Methods | Randomisation: stated as randomised. Allocation concealment: unclear. Blinding: double. Intention to treat analysis: not performed. Power calculation: not performed. Study setting: single centre ‐ Paris, France. Withdrawals: none. Cancelled cycles: <10%. |
|
| Participants | Number of women n = 35 (12 growth hormone 4IU, 11 growth hormone 8IU, 12 placebo). Inclusion criteria: women were <39 years old with normal hormonal status and history of oocyte dysmorphia defined by <50% of abnormal oocyte at previous attempts. |
|
| Interventions | Intervention: four or eight IU sub cutaneous. Induction protocol: unclear. Dose of human chorionic gonadotropin: 1000 IU IM when at least two follicles were >16mm in diameter. | |
| Outcomes | Pregnancy rate. | |
| Notes | Thirty‐five women in total were included in Hazout 2003 and they were divided into three groups, placebo, growth hormone four IU and growth hormone eight IU. Since only two groups could be compared for the table of comparisons the two growth hormone groups were separated and compared with half the placebo data for the meta‐analysis but throughout the text the trial is referred to singly as Hazout 2003. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated as randomised. No other details |
| Allocation concealment? | Unclear risk | Not stated within the text |
| Blinding? All outcomes | Low risk | Double blinded |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | High risk | No adverse effects reported |
Hazout 2003 4 IU.
| Methods | Randomisation: stated as randomised. Allocation concealment: unclear. Blinding: double. Intention to treat analysis: not performed. Power calculation: not performed. Study setting: single centre ‐ Paris, France. Withdrawals: none. Cancelled cycles: <10% |
|
| Participants | Number of women n = 35 (12 growth hormone four IU, 11 growth hormone 8IU, 12 placebo). Inclusion criteria: Women were <39 years old with normal hormonal status and history of oocyte dysmorphia defined by <50% of abnormal oocyte at previous attempts. |
|
| Interventions | Intervention: four or eight IU sub cutaneous. Induction protocol: unclear. Dose of human chorionic gonadotropin: 1000 IU IM when at least two follicles were >16mm in diameter. | |
| Outcomes | Pregnancy rate. | |
| Notes | Same trial as Hazout 2003 but refers to women randomised to growth hormone four IU treatment arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated as randomised |
| Allocation concealment? | Unclear risk | Not stated within the text |
| Blinding? All outcomes | Low risk | Doubleblinded |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | High risk | No adverse effects reported |
Hazout 2003 8 IU.
| Methods | Randomisation: stated as randomised. Allocation concealment: unclear. Blinding: double. Intention to treat analysis: not performed. Power calculation: not performed. Study setting: single centre ‐ Paris, France. Withdrawals: none. Cancelled cycles: <10%. |
|
| Participants | Number of women n = 35 (12 growth hormone four IU, 11 growth hormone eight IU, 12 placebo). Inclusion criteria: women were <39 years old with normal hormonal status and history of oocyte dysmorphia defined by <50% of abnormal oocyte at previous attempts. |
|
| Interventions | Intervention: four or eight IU sub cutaneous. Induction protocol: unclear. Dose of human chorionic gonadotropin: 1000 IU IM when at least two follicles were >16mm in diameter. | |
| Outcomes | Pregnancy rate. | |
| Notes | Same trial as Hazout 2003. but refers to women randomised to growth hormone 8 IU treatment arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated as randomised |
| Allocation concealment? | Unclear risk | Not stated within the text |
| Blinding? All outcomes | Low risk | Double blinded |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | High risk | No adverse effects reported |
Kueuk 2008.
| Methods | Randomisation: computer generated randomisation. Allocation concealment: sealed envelopes. Blinding: triple. Intention to treat analysis: not performed. Power calculation: not performed. Study setting: single centre ‐ Bursa, Turkey. Withdrawals: none. Cancelled cycles: <10%. | |
| Participants | Number of women n= 61 (31 growth hormone, 30 placebo). Inclusion criteria: women who responded poorly to high dose gonadotrophin treatment in their first cycles in the same centre. Cause of subfertility: Not stated. |
|
| Interventions | Intervention: growth hormone 12IU sub cutaneous from day 21 of preceding cycle along with GnRHa, until the day of human chorionic gonadotropin. Treatment Protocol: Long GnRHa/FSH/hMG protocol used. Dose of human chorionic gonadotropin: 10000 IU when sat least 1 follicle was > 17mm in diameter. | |
| Outcomes | Clinical pregnancy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated randomisation |
| Allocation concealment? | Unclear risk | Sealed envelopes. No details as to whether opaque |
| Blinding? All outcomes | Low risk | Triple blinded |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | High risk | No adverse effects reported |
Owen 1991.
| Methods | Randomisation: Two randomisation lists were made with 20 women on each list and block randomised into blocks of four. Allocation Concealment: method unclear Blinding: double‐blind Trial design: Parallel. Analysis: No power calculation or intention to treat analysis performed. Study setting: single centre, location London. Withdrawals: none (<10%). Cancelled Cycles: <10%. | |
| Participants | Number of women:n= 25 (13 growth hormone, 12 placebo). Inclusion criteria: one or more previous IVF cycles with poor response, defined as fewer than six oocytes retrieved from which fewer than three embryos developed. Cause of subfertility: 18 of 25 women found to have polycystic ovaries on ultrasound. Age: <38 |
|
| Interventions | Intervention: growth hormone 24 IU intramuscular (IM), days 1, 3, 5, 7, 9, and 11 of hMG treatment, during long GnRHa protocol, vs placebo given IM on same cycle days as active treatment groups. Dose of human chorionic gonadotropin: 5000 IU | |
| Outcomes | Live birth rate, pregnancy rate, adverse effects (multiple pregnancy and ectopic pregnancy). | |
| Notes | Nature of placebo not described. Follicular fluid IG1 increased by 27% with growth hormone treatment. The data from Jacobs 1995 is also presented in Owen 1991. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Two randomisation lists were made with 20 women on each list and block randomised into blocks of four |
| Allocation concealment? | Unclear risk | Not stated within the text |
| Blinding? All outcomes | Low risk | Double blinded |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | High risk | Nature of placebo not described. Follicular fluid IG1 increased by 27% with growth hormone treatment |
Suikkari 1996.
| Methods | Randomisation: Stated as randomised. Allocation Concealment: unclear. Blinding: Double blind. Trial Design: Parallel. Analysis: No power calculation and no intention to treat analysis performed Study Setting: two centres. Analysis: No power calculation or intention‐to‐treat analysis performed. Withdrawals: Withdrawals: < 10% . Cancelled Cycles: >10% (therefore include in meta‐analysis but perform sensitivity analysis). |
|
| Participants | Number of women: n= 22 (10 growth hormone 4 IU, 6 growth hormone 12 IU, 6 placebo) Inclusion Criteria: previous poor response in more than or equal to two assisted cycles. Definition of poor Response: < or equal to two oocytes retrieved or > or equal to 48 AMP hMG consumed in a stimulation cycle. Cause of subfertility: tubal (n=10), endometriosis (n=1), male factor (n=2), idiopathic (n=9). Age 25‐40 years. |
|
| Interventions | Intervention: six women received 12 IU growth hormone and 10 women received four IU growth hormone daily SC from day three of spontaneous menstrual cycle. Study Protocol: A boost "flare‐up" protocol was used for ovarian stimulation. On day two of spontaneous menstrual cycle leuprolide acetate was administered SC 0.75mg in the morning. On day three gonadotrophin Metrodin was started at 300IU SC for four days then adjusted according to serum E2 and follicular growth. Dose of human chorionic gonadotropin 5000 IU IM given when the largest follicle(s) reached a diameter of 18 to 20mm. | |
| Outcomes | Live birth rate, pregnancy rate, embryo transfer and adverse effects (multiple pregnancy). | |
| Notes | Twenty two women in total were included in Suikkari 1996 and they were divided into three groups, placebo, growth hormone four IU and growth hormone 12IU. Since only two groups could be compared for the table of comparisons the two growth hormone groups were separated and compared with half the placebo data for the meta‐analysis but throughout the text the trial is referred to singly as Suikkari 1996. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated as randomised |
| Allocation concealment? | Unclear risk | Not stated within the text |
| Blinding? All outcomes | Low risk | Double blinded |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | High risk | Cancelled Cycles: >10% (therefore include in meta‐analysis but perform sensitivity analysis). |
Suikkari 1996 12 IU.
| Methods | Randomisation: Stated as randomised. Allocation concealment: unclear. Blinding: Double blind. Trial Design: Parallel. Analysis: No power calculation and no intention to treat analysis performed Study Setting: two centres. Analysis: No power calculation or intention to treat analysis performed. Withdrawals: Withdrawals: < 10% . Cancelled Cycles: >10% (therefore include in meta‐analysis but perform sensitivity analysis). |
|
| Participants | Number of women : n=22 (10 growth hormone four IU, six growth hormone 12 IU, six placebo) Inclusion Criteria: previous poor response in more than or equal to two assisted cycles. Definition of poor Response: < or equal to two oocytes retrieved or > or equal to 48 AMP hMG consumed in a stimulation cycle. Cause of subfertility: tubal (n=10), endometriosis (n=1), male factor (n=2), idiopathic (n=9). Age 25‐40 years. | |
| Interventions | Intervention: six women received 12 IU growth hormone and 10 women received 4 IU growth hormone daily SC from day three of spontaneous menstrual cycle. Study Protocol: A boost "flare‐up" protocol was used for ovarian stimulation. On day two of spontaneous menstrual cycle leuprolide acetate was administered SC 0.75mg in the morning. On day three gonadotrophin Metrodin was started at 300IU SC for four days then adjusted according to serum E2 and follicular growth. Dose of human chorionic gonadotropin 5000 IU IM given when the largest follicle(s) reached a diameter of 18 to 20mm. | |
| Outcomes | Live birth rate, pregnancy rate, embryo transfer and adverse effects (multiple pregnancy). | |
| Notes | Same trial as Suikkari 1996 but refers to women randomised to growth hormone 12 IU treatment arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated as randomised |
| Allocation concealment? | Unclear risk | Not stated within the text |
| Blinding? All outcomes | Low risk | Double blinded |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | High risk | Cancelled Cycles: >10% (therefore include in meta‐analysis but perform sensitivity analysis) |
Suikkari 1996 4 IU.
| Methods | Randomisation: Stated as randomised. Allocation concealment: unclear. Blinding: Double blind. Trial Design: Parallel. Analysis: No power calculation and no intention to treat analysis performed Study Setting: 2 centres. Analysis: No power calculation or intention to treat analysis performed. Withdrawals: Withdrawals: < 10% . Cancelled Cycles: >10% (therefore include in meta‐analysis but perform sensitivity analysis). |
|
| Participants | Number of women: 22 (10 growth hormone 4 IU, 6 growth hormone 12 IU, 6 placebo) Inclusion Criteria: previous poor response in more than or equal to 2 assisted cycles. Definition of poor Response: < or equal to 2 oocytes retrieved or > or equal to 48 AMP hMG consumed in a stimulation cycle. Cause of subfertility: tubal (n=10), endometriosis (n=1), male factor (n=2), idiopathic (n=9). Age 25‐40 years. |
|
| Interventions | Intervention: 6 women received 12 IU growth hormone and 10 women received 4 IU growth hormone daily SC from day 3 of spontaneous menstrual cycle. Study Protocol: A boost "flare‐up" protocol was used for ovarian stimulation. On day 2 of spontaneous menstrual cycle leuprolide acetate was administered SC 0.75mg in the morning. On day 3 gonadotrophin Metrodin was started at 300IU SC for 4 days then adjusted according to serum E2 and follicular growth. Dose of human chorionic gonadotropin 5000 IU IM given when the largest follicle(s) reached a diameter of 18‐20mm. | |
| Outcomes | Live birth rate, pregnancy rate, embryo transfer and adverse effects (multiple pregnancy). | |
| Notes | Same trial as Suikkari 1996 but refers to women randomised to growth hormone 4 IU treatment arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated as randomised |
| Allocation concealment? | Unclear risk | Not stated within the text |
| Blinding? All outcomes | Low risk | Double blinded |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | High risk | Cancelled Cycles: >10% (therefore include in meta‐analysis but perform sensitivity analysis) |
Tapanainen 1992.
| Methods | Randomisation: Stated as randomised, method unclear. Allocation concealment: trial codes kept in sealed envelopes until the study was completed. Blinding: double‐blind Trial design: Parallel. Analysis: Power calculation not done, no intention to treat analysis but no withdrawals. Study Setting: single centre. Finland Withdrawals: none (<10%). Cancelled cycles: <10%. |
|
| Participants | Number of women randomised: n=38 (19 growth hormone, 19 placebo). Cause of Subfertility: normally cycling women with unexplained infertility, tubal infertility or mild to moderate endometriosis. Age: 27‐37. | |
| Interventions | Intervention: growth hormone 24 IU IM beginning on cycle day four, then every 2 days until human chorionic gonadotropin, vs sterile saline IM on same cycle days. Treatment Protocol: Short GnRHa protocol used for ovulation induction, 300µg BA 3 times daily on cycle days 1‐4. Three ampoules of hMG given IM on day 4 and then 150‐223 IU daily until human chorionic gonadotropin injection. 5000 IU human chorionic gonadotropin given. Clinical Pregnancy Diagnosis: USS at six weeks gestation | |
| Outcomes | Live birth rate and adverse effects (multiple pregnancies) | |
| Notes | There were two parts to this trial, A and B. Only data from part A was included as part B studied the effect of growth hormone on gene expression of steroidogenic enzymes in granulosa cells and the women were not followed up for live birth or pregnancy data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated as randomised |
| Allocation concealment? | Unclear risk | Trial codes kept in sealed envelopes until the end of the study, no details as to whether centralised or envelopes opaque |
| Blinding? All outcomes | Low risk | Double blinding |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | Low risk | Report both live birth rate and adverse effects |
Tesarik 2005.
| Methods | Randomisation: truly randomised, computer generated random number tables. Allocation concealment: clear, opaque envelopes. Blinding: double‐blinded. Analysis: Power calculation performed and intention to treat analysis not performed. Study setting: multi‐centre, Spain and France. Withdrawals: none. Cancelled cycles: <10%. |
|
| Participants | Number of women: 100 (50, growth hormone, 50 placebo). Inclusion criteria: women >40 years old asking for an assisted reproduction attempt by ICSI were assessed for eligibility. | |
| Interventions | Intevention: growth hormone 8IU Subcut. Treatment Protocol: Long. Dose of human chorionic gonadotropin: 25mg when at least 1 follicle measured >18mm in diameter. | |
| Outcomes | Live birth rate, pregnancy rate. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random number tables |
| Allocation concealment? | Low risk | Opaque envelopes |
| Blinding? All outcomes | Low risk | Double blinded |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | High risk | No details of adverse effects reported |
Younis 1992.
| Methods | Randomisation: Prospectively randomised, method unclear. Allocation concealment: allocation not revealed until all outcome measures were calculated and comparison between the two groups had been performed. Blinding: double‐blind. Study design: placebo controlled trial. Sensitivity analysis: No power calculation or intention to treat analysis performed. Study setting: single centre, location Israel. Withdrawals: none (< 10%). Cancelled Cycles: <10%. |
|
| Participants | Number of women randomised: n= 42 (20 growth hormone, 22 placebo). Cause of Subfertility: Ovulating women with mechanical factor infertility. Normal serum FSH, LH, PRL, T and DHEAS. Normal semen (WHO criteria). Exclusion Criteria: ? Age: < or equal to 38 years | |
| Interventions | Intervention: growth hormone 12 IU SC on days 1, 3, 5, and 7 of hMG treatment vs Mannitol 30 mg SC on same cycle days. Treatment Protocol: All women received GnRHa/hMG 0.5mg/day from day 21 of previous cycle ovulation induction protocol. | |
| Outcomes | Pregnancy rate, oocyte retrieval, embryo transfer, ampoules of Gonadotrophin used and adverse effects (multiple pregnancy). | |
| Notes | Mannitol chosen as placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated as randomised |
| Allocation concealment? | Unclear risk | Allocation not revealed until all outcomes calculated and comparisons between groups performed |
| Blinding? All outcomes | Low risk | Double blinded |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | Low risk | |
Zhuang 1994.
| Methods | Randomisation: stated as randomised, method unclear. Allocation concealment: unclear of method Blinding: Outcome assessors were blind to treatment allocation. Study Design: Parallel. Study Setting: unclear. Analysis; Power calculation done. Withdrawals; none. Cancelled Cycles; none. |
|
| Participants | Number of women randomised: n=27 (12 growth hormone, 15 control). Definition of poor response: not provided Inclusion Criteria: previous sub‐optimal response to hyperstimulation cycles in IVF. Exclusion Criteria: Cause of subfertility: tubal factor or unexplained. Age: growth hormone 33.2 +/‐3.9, Placebo 32.3 +/‐3.9. |
|
| Interventions | Intervention: growth hormone 12 IU IM on alternate days. Treatment Protocol: GnRH‐a (Buserelin nasal spray) from day 21 of previous menstrual cycle to day of human chorionic gonadotropin injection (do not know dose of GnRH‐a) 2 IU hMG given on alternate days for 12 days (at same time as growth hormone). Dose of human chorionic gonadotropin: 10000 iu. | |
| Outcomes | Live birth rate and pregnancy rate. | |
| Notes | Some information will have been stated in the trial but was not translated. The sections that were translated were kindly done so by Teresa Gu. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated as randomised |
| Allocation concealment? | Unclear risk | Not stated within the text |
| Blinding? All outcomes | Low risk | Single |
| Free of selective reporting? | Low risk | There is no indication the study has reported outcomes selectively |
| Free of other bias? | High risk | No details of adverse effects reported |
BA: Buserelin Acetate E2: Oestrogen Only outcomes relevant to the review were stated in the table of included studies GnRH‐a: Gonadotrophin releasing hormone agonist
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blumenfeld 1994 | During the 2009 update additional data was sort to clarify which women included in the trial received which method of assisted conception and the definition of poor responder used. |
| Busacca 1996 | Method of assisted conception used was not IVF but artificial insemination‐husband or GIFT. |
| Homburg 1990 | Not stated as randomised, no useful outcomes reported. |
| Homburg 1990b | Women did not undergo IVF. |
| Homburg 1995 | Women did not undergo IVF. |
| Howles 1999 | Intervention is growth hormone releasing factor not growth hormone. |
| Hughes 1994 | Any women who failed to produce 3 follicles greater than 20mm were cancelled. These women were not included in the analysis. This unpublished information could not be obtained from the author. Hughes 1992 and Huang 1993 are the same trial as Hughes 1994. |
| Jacobs 1995 | Only concerns ovulation induction, not IVF. |
| Landolfi 1994 | Only concerns ovulation induction, not IVF. |
| Owen 1991b | There are two publications for this trial. The analysis used women randomised to receive growth hormone in the trial and retrospective cases of women who had also received growth hormone in the past. |
| Rinehart 1999 | Allocation was stated as "alternating randomisation", suggesting allocation to groups by alternation, not randomisation. |
| Schoolcraft 1997 | Both treatment groups received the same dose of growth hormone, the intervention was oral contraceptive. |
| Tulandi 1993 | Method of assisted conception was intra uterine insemination not IVF. |
Differences between protocol and review
The authors of the protocol were different from the review, additional authors were included in the 2009 update: G Ahmad, J Brown, JMN Duffy, L Nardo, I Salim and AJ Watson.
Contributions of authors
D Kotarba, J Kotarba, and E Hughes prepared the original version of this review published in 1995. The 2003 update of the review was prepared by K Harper and M Proctor. The 2009 update of the review was prepared by G Ahmad, J Brown, JMN Duffy and L Nardo, I Salim and AJ Watson
Sources of support
Internal sources
Dept of Obstetrics and Gynaecology, University of Auckland, New Zealand.
External sources
-
Department of Health, UK.
$5,000 initiative fund.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Bergh 1994 {published data only}
- Bergh C, Carlstrom K, Selleskog U, Hillensjo T. Effect of growth hormone on follicular fluid androgen levels in patients treated with gonadotropins before in vitro fertilization. European Journal of Endocrinology 1996;134:190‐6. [DOI] [PubMed] [Google Scholar]
- Bergh C, Nilsson L, Hillensjo T, Borg G, Wikland M, Hamgerger L. Adjuvant growth hormone treatment during in vitro fertilization: a randomized, placebo‐controlled study. Fertility & Sterility 1994;62:113‐20. [PubMed] [Google Scholar]
Dor 1995 {published data only}
- Dor J, Seidman DS, Amudia E, Bider D, Levran D, Mashiach S. Adjuvant growth hormone therapy in poor responders to in‐vitro fertilization: a prospective randomized placebo‐controlled double‐blind study. Human Reproduction 1995;10:40‐3. [DOI] [PubMed] [Google Scholar]
Hazout 2003 {published data only}
- Hazout A, Junca AM. A pilot, prospective, randomised, double blind, placebo‐controlled study to compare protocols for ovarian stimulation with r‐hRSH (Gonal‐F) combined with two different doses of r‐hGH or placebo in patients with oocyte dysmorphy undergoing ICSI. Human Reproduction 2003;18(Supplement 1):299‐212. [Google Scholar]
Hazout 2003 4 IU {published data only}
- Hazout A, Junca AM. A pilot, prospective, randomised, double blind, placebo‐controlled study to compare protocols for ovarian stimulation with r‐hRSH (Gonal‐F) combined with two different doses of r‐hGH or placebo in patients with oocyte dysmorphy undergoing ICSI. Human Reproduction 2003;18(Supplement 1):299‐212. [Google Scholar]
Hazout 2003 8 IU {published data only}
- Hazout A, Junca AM. A pilot, prospective, randomised, double blind, placebo‐controlled study to compare protocols for ovarian stimulation with r‐hRSH (Gonal‐F) combined with two different doses of r‐hGH or placebo in patients with oocyte dysmorphy undergoing ICSI. Human Reproduction 2003;18(Supplement 1):299‐212. [Google Scholar]
Kueuk 2008 {published data only}
- Kucuk T, Kozinoglu H, Kaba A. Growth hormone co‐treatment within a GnRH agonist long protocol in patients with poor ovarian response:a prospective, randomized, clinical trial. Journal of Assisted Reproduction and Genetics 2008;25:123‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Owen 1991 {published data only}
- Jacobs HS. Growth hormone and ovulation: Is there an indication for treatment of infertile women with growth hormone?. Hormone Research 1992;38:14‐21. [DOI] [PubMed] [Google Scholar]
- Owen EJ, Ostergaard H, Shoham Z, Jacobs HS, Mason BA. Cotreatment with growth hormone, after pituitary suppression, for ovarian stimulation in in vitro fertilization: a randomized, double‐blind, placebo‐control trial. Fertility & Sterility 1991;56:1104‐10. [DOI] [PubMed] [Google Scholar]
Suikkari 1996 {published data only}
- Suikkari AM, MacLachlan V, Koistinen R, Seppälä M, Healy DL. Double‐blind placebo controlled study: human biosynthetic growth hormone for assisted reproductive technology. Fertility & Sterility 1996;65(4):800‐5. [DOI] [PubMed] [Google Scholar]
Suikkari 1996 12 IU {published data only}
- Suikkari AM, MacLachlan V, Koistinen R, Seppälä M, Healy DL. Double‐blind placebo controlled study: human biosynthetic growth hormone for assisted reproductive technology. Fertility & Sterility 1996;65(4):800‐5. [DOI] [PubMed] [Google Scholar]
Suikkari 1996 4 IU {published data only}
- Suikkari AM, MacLachlan V, Koistinen R, Seppälä M, Healy DL. Double‐blind placebo controlled study: human biosynthetic growth hormone for assisted reproductive technology. Fertility & Sterility 1996;65(4):800‐5. [DOI] [PubMed] [Google Scholar]
Tapanainen 1992 {published data only}
- Tapanainen J, Orava M, Martikainen H, Ruokonen A, Voutilainen R, Ronnberg L. Effect of growth hormone administration on human ovarian function and steroidogenic gene expression in granulosa‐luteal cells. Fertility & Sterility 1992;58:726‐32. [DOI] [PubMed] [Google Scholar]
Tesarik 2005 {published data only}
- Tesarik J, Hazout A, Mendoza C. Improvement of delivery and live birth rates after ICSI in women aged>40 years by ovarian co‐stimulation with growth hormone. Human Reproduction 2005;20(9):2536‐2541. [DOI] [PubMed] [Google Scholar]
Younis 1992 {published data only}
- Younis JS, Dorembus D, Simon A, Schenker JG, Koren R, Laufer N. The effect of growth hormone supplementation on in vitro fertilization outcome: a prospective randomized placebo‐controlled double‐blind study. Fertility & Sterility 1992;58:575‐80. [DOI] [PubMed] [Google Scholar]
- Younis JS, Ezra Y, Brzezinnski, Fibich T, Schenker JG, Laufer N. The effect of growth hormone on granulosa cell function during in‐vitro fertilization. Human Reproduction 1993;8(10):1588‐92. [DOI] [PubMed] [Google Scholar]
Zhuang 1994 {published data only}
- Zhuang GL, Wong SX, Zhou CQ. The effect of co‐administration of low dosage growth hormone and gonadotropin for ovarian hyperstimulation in vitro fertilization and embryo transfer. Chung‐Hua Fu Chan Ko Tsa Chih (Chinese Journal of Obstetrics and Gynaecology) 1994;29(8):471‐4. [PubMed] [Google Scholar]
References to studies excluded from this review
Blumenfeld 1994 {published data only}
- Blumenfeld Z, Amit T. The role of growth hormone (GH), GH‐receptor and GH‐binding protein in reproduction and ovulation induction. Journal of Pediatric Endocrinology & Metabolism 1996;9:145‐62. [PubMed] [Google Scholar]
- Blumenfeld Z, Amit T. The role of growth hormone in ovulation induction. Annals of Medicine 1994;26:249‐54. [DOI] [PubMed] [Google Scholar]
- Blumenfeld Z, Dirnfeld M, Gonen Y, Abramovici H. Growth hormone co‐treatment for ovulation induction may enhance conception in the co‐treatment and succeeding cycles, in clonidine negative but not clonidine positive patients. Human Reproduction 1994;9:209‐13. [DOI] [PubMed] [Google Scholar]
Busacca 1996 {published data only}
- Busacca M, Fusi MF, Brigante C, Bonzi V, Gonfiantini C, Vignali M, et al. Use of growth hormone‐releasing factor in ovulation induction in poor responders. Journal of Reproductive Medicine 1996;41(9):699‐703. [PubMed] [Google Scholar]
Homburg 1990 {published data only}
- Homburg R, West C, Torresani T, Jacobs HS. A comparative study of single‐dose growth hormone as an adjuvant to gonadotrophin treatment for ovulation induction. Clinical Endocrinology 1990;32:781‐5. [DOI] [PubMed] [Google Scholar]
Homburg 1990b {published data only}
- Homburg R, West C, Torresani T, Jacobs HS. Cotreatment with human growth hormone and gonadotropins for induction of ovulation: a controlled clinical trial. Fertility & Sterility 1990;53(2):254‐60. [DOI] [PubMed] [Google Scholar]
Homburg 1995 {published data only}
- Homburg R, Levy T, Ben‐Rafael Z. Adjuvant growth hormone for induction of ovulation with gonadotrophin‐releasing hormone agonist and gonadotrophins in polycystic ovary syndrome: a randomized, double‐blind, placebo controlled trial. Human Reproduction 1995;10:2550‐3. [DOI] [PubMed] [Google Scholar]
Howles 1999 {published data only}
- Howles CM, Loumaye E, Germond M, Yates R, Brinsden P, Healy D, Bonaventura LM, Strowitzki T. Does growth hormone‐releasing factor assist follicular development in poor responder patients undergoing ovarian stimulation for in‐vitro fertilisation?. Human Reproduction 1999;14(8):1939‐1943. [DOI] [PubMed] [Google Scholar]
Hughes 1994 {published data only}
- Huang ZH, Baxter RC, Hughes SM, Matson PL, Lieberman BA, Morris ID. Supplementary growth hormone treatment of women with poor ovarian response to exogenous gonadotrophins: changes in serum and follicular fluid insulin‐like growth factor‐1 (IGF‐1) and IGF binding protein‐3 (IGFBP‐3). Human Reproduction 1993;8:850‐7. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Huang ZH, Morris ID, Matson PL, Buck P, Lieberman BA. A double blind cross over controlled study to evaluate the effect of human biosynthetic growth hormone on ovarian stimulation in previous IVF poor responders. Journal of Reproductive Fertility 1992;96:21. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Huang ZH, Morris ID, Matson PL, Buck P, Lieberman BA. A double‐blind cross‐over controlled study to evaluate the effect of human biosynthetic growth hormone on ovarian stimulation in previous poor responders to in‐vitro fertilization. Human Reproduction 1994;9:13‐8. [DOI] [PubMed] [Google Scholar]
Jacobs 1995 {published data only}
- Jacobs HS, Shoham Z, Schachter M, Braat DDM, Franks S, Hamilton‐Fairley D. Cotreatment with growth hormone and gonadotropin for ovulation induction in hypogonadotropic patients: A prospective, randomized, placebo‐controlled, dose‐response study. Fertility & Sterility 1995;64(5):917‐23. [PubMed] [Google Scholar]
Landolfi 1994 {published data only}
- Landolfi L, Marra V, Gallina N. Ovulation induction with growth hormone and GnRH in polycystic ovarian disease. Rassegna Internazionale di Clinica e Terapia 1994;74(12):529‐32. [Google Scholar]
Owen 1991b {published data only}
- Owen EJ, Torresani T, West C, Mason BA, Jacobs HS. Serum and follicular fluid insulin like growth factors I and II during growth hormone co‐treatment for in‐vitro fertilization and embryo transfer. Clinical Endocrinology 1991;35:3217‐34. [DOI] [PubMed] [Google Scholar]
- Owen EJ, West C, Mason BA, Jacobs HS. Cotreatment with growth hormone of sub‐optimal responders in IVF‐ET. Human Reproduction 1991;6:524‐8. [DOI] [PubMed] [Google Scholar]
Rinehart 1999 {published data only}
- Rinehart JS. Randomized, prospective trial comparing the addition of growth hormone for the ovulation induction of "poor responders" in IVF. Fertility & Sterility 1999;72(3):S91. [Google Scholar]
Schoolcraft 1997 {published data only}
- Schoolcraft W, Schlenker T, Gee M, Stevens J, Wagley L. Improved controlled ovarian hyperstimulation in poor responder in vitro fertilization patients with a microdose follicle‐stimulating hormone flare, growth hormone protocol. Fertility & Sterility 1997;67(1):93‐7. [DOI] [PubMed] [Google Scholar]
Tulandi 1993 {published data only}
- Tulandi T, Falcone T, Guyda H, Hemmings R, Billiar R, Morris D. Effects of synthetic growth hormone‐releasing factor in women treated with gonadotrophin. Human Reproduction 1993;8(4):525‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Adashi 1985
- Adashi EY, Resnick CE, DErcole AJ, Svoboda ME, Wyke J. Insulin‐like growth factors as intraovarian regulators of granulosa cell growth and function. Endocrinology Review 1985;6:400‐20. [DOI] [PubMed] [Google Scholar]
Blumenfeld 1996
- Blumenfeld Z, Amit T. The role of growth hormone (GH), GH‐receptor and GH‐binding protein in reproduction and ovulation induction. Journal of Pediatric Endocrinology & Metabolism 1996;9:145‐62. [PubMed] [Google Scholar]
Cahill 2002
- Cahill D, Wardle PG. Management of Infertility. BMJ: British Medical Journal 2002;325(7354):28‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erickson 1989
- Erickson GF, Gabriel VG, Magoffin DA. Insulin‐like factor‐I regulates aromataze activity in human granulosa and granulosa luteal cells. Journal of Clinical Endocrinological Metabolism 1989;69:716‐24. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration. Wiley, 2008. [Google Scholar]
Homburg 1988
- Homburg R, Eshel A, Abdulla HI, Jacobs HS. Growth hormone facilitates ovulation induction by gonadotrophins. Clinical Endocrinology 1988;29:113‐8. [DOI] [PubMed] [Google Scholar]
Hsu 1987
- Hsu DJ, Hammond JM. Concomitant effects of growth hormone on secretion of insulin‐like growth factor I and progesterone by cultured porcine granulosa cells. Endocrinology 1987;121(4):1343‐8. [DOI] [PubMed] [Google Scholar]
Kyrou 2009
- Kyrou D, Kolibianakis EM, Venetis CA, Papanikolaou EG, Bontis J, Tarlatzis BC. How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: a systematic review and meta‐analysis. Fertility and Sterility 2009;91(3):749‐766. [DOI] [PubMed] [Google Scholar]
Li 2005
- Li P, Mah D, Lim K, Sprague S, Bhandari M. Randomization and concealment in surgical trials: a comparison between orthopaedic and non‐orthopaedic randomized trials. Archives of Orthopeadic and Trauma Surgery 2005;125(1):1434‐3916. [DOI] [PubMed] [Google Scholar]
Mason 1990
- Mason HD, Martikainen H, Beard RW, Anyaoku V, Franks S. Direct gonadotrophic effect of growth hormone on oestradiol production by human granulosa cell in vitro. Journal of Endocrinology 1990;126:R1‐R2. [DOI] [PubMed] [Google Scholar]
Yoshimura 1996
- Yoshimura Y, Ando M, Nagamatsu S, Iwashita M, Adachi T, Sueoka K. Effects of insulin‐like growth factor‐I on follicle growth, oocyte maturation, and ovarian steroidogenesis and plasminogen activator activity in the rabbit. Biology of Reproduction 1996;55(1):152‐60. [DOI] [PubMed] [Google Scholar]


