Abstract
Background:
Studies suggest HIV+ patients have an increased risk of coronary artery disease, yet little is known about the histopathology, severity, or distribution of lesions.
Methods:
The coronary arteries of 66 deceased AIDS patients and 19 HIV− controls (age <55) were dissected and graded for percent luminal stenosis by intimal lesions, percent of intima involved with lipid, and extent of intimal calcification on a scale of 0 to 3. Medical histories, antiretroviral therapies, and coronary artery disease (CAD) risk factors were reviewed.
Results:
HIV+ patients were older than controls (p=0.06), and more were male (p=0.02). Thirty-five percent of HIV+ patients had stenosis ≥75% of at least one artery. Compared to controls, HIV+ patients had 3 times greater odds of stenosis ≥75%, controlling for age and sex (1-sided p=0.03). Older age and male sex were also risk factors (1-sided p<0.001). HIV seropositivity was associated with increased plaque lipid content (1-sided p=0.02) and calcification (1-sided p=0.08). Duration of HIV infection, antiretroviral therapy, and immune status did not predict severe disease in multivariate analyses. Previously unreported patterns of dystrophic calcification were observed in HIV+ patients and older controls.
Conclusions:
Young to middle-aged patients dying from advanced AIDS have atherosclerotic coronary artery disease that may result in luminal narrowing, heavy calcification, and high plaque lipid content. The pattern of disease, location of lesions, and plaque composition are typical of atherosclerosis in HIV− patients. No relationship between antiretroviral therapies and atherosclerosis was seen in this small study of heavily-treated patients.
Keywords: AIDS, atherosclerosis, calcium, coronary disease, pathology
Summary:
The pattern, location, and composition of coronary lesions in AIDS patients are typical of atherosclerosis in HIV-negative patients. Controlling for age and sex, AIDS patients are more likely to have lesions resulting in stenosis >75% and higher plaque lipid content. Heavy calcification and novel patterns of dystrophic calcification are also observed.
Background/Introduction:
Highly active antiretroviral therapy (HAART) has greatly reduced the morbidity and mortality associated with HIV infection and AIDS.(1) Yet, long-term survivors of this disease are susceptible to enduring effects of HIV infection and treatment.
Several studies have linked HIV infection and protease inhibitor (PI) exposure as part of HAART with myocardial infarction (MI), coronary artery disease (CAD), endothelial dysfunction, and increased carotid intima-media thickness.(2–6) This increase in coronary events and atherosclerosis may be due to PIs, which have been associated with hyperlipidemia and insulin resistance.(7) Alternatively, HIV itself may be atherogenic. HIV-infected macrophages, for example, have impaired cholesterol efflux and accumulate substantial amounts of lipid similar to macrophage foam cells; these HIV-infected cells have been demonstrated in atherosclerotic plaques.(8) Progressive HIV disease is associated with immune activation, T-cell proliferation, proinflammatory cytokines, endothelial markers, and elevated C-reactive protein.(3, 9, 10) Chronic inflammation and immune activation have been shown to contribute to accelerated atherosclerosis.(11–14)
Though there has been much research in this area, little is known about the histopathology of coronary arteriosclerosis in HIV-infected patients. Just two autopsy studies on this subject have been reported in adults, totaling 15 patients.(15, 16) Among these patients, ages 23-32, there was a high burden of arteriosclerotic disease. Further, the authors felt the appearance of the lesions was similar to that of cardiac transplant vasculopathy. This condition is thought to be an accelerated, immune-mediated form of CAD associated with chronic rejection, and it is linked to increased rates of clinical coronary events. Transplant vasculopathy has a characteristic appearance featuring extensive, concentric fibromuscular hyperplasia of the intima extending through both proximal and distal portions of the epicardial coronary arteries and smaller intramyocardial arteries (Figure 1, Panel A). Transplant vasculopathy is thought to be caused by activated T-cells and inflammatory cytokines. (17, 18) Typical atheromas also occur. If HIV-associated arteriosclerosis has a similar histologic appearance, this might suggest an analogous, immune-mediated pathophysiology.
Fig 1:
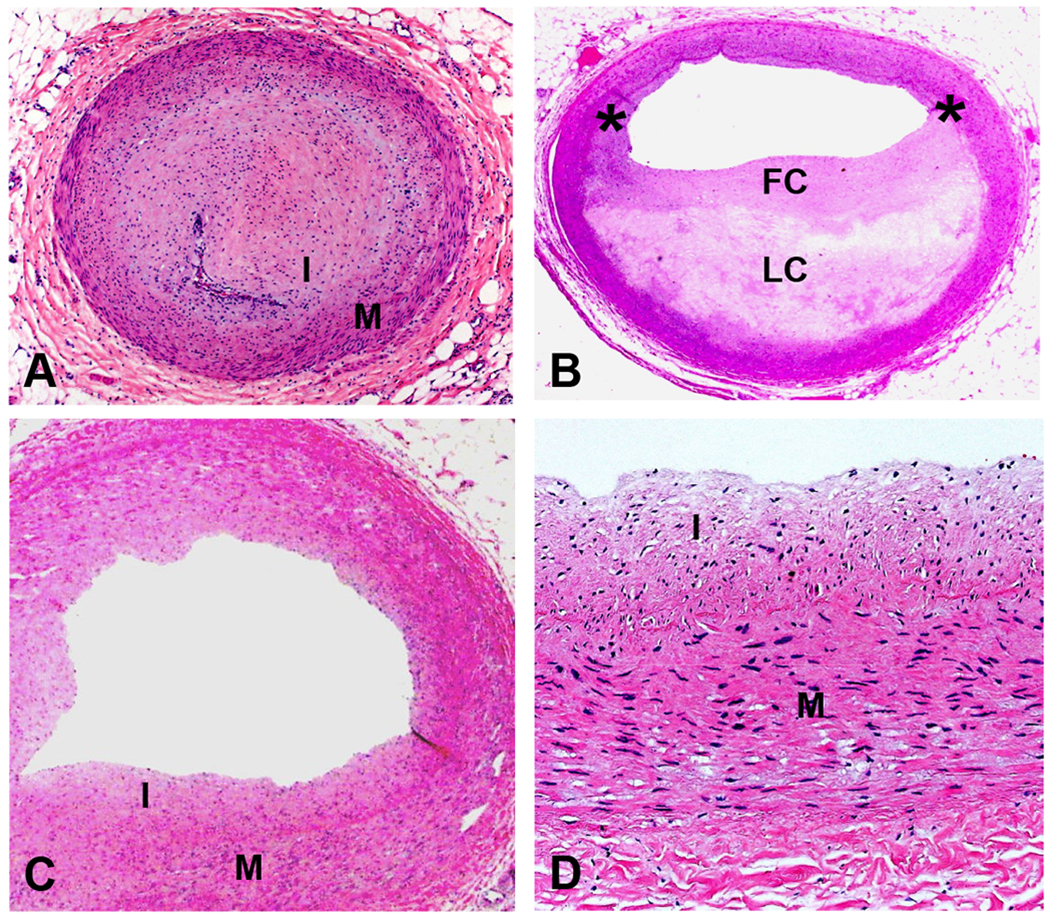
Definitions: A) Fibromuscular intimal hyperplasia from a case of transplant coronary artery disease showing a normal media (M) with overlying concentric intimal thickening (I) causing complete luminal occlusion. Some inflammatory cells are also present; B) Atherosclerotic plaque defined as an eccentric lesion with a lipid core (LC) covered by a fibrous cap (FC) (asterisks indicate shoulder region of the plaque). The region opposite the eccentric plaque exhibits normal histology; C) Section of artery with adaptive intimal thickening also showing a normal media (M) with mild, non-occlusive intimal thickening (I); and D) Higher magnification of C showing intima composed of smooth muscle cells and collagen (all H&E, A, B, and C orig mag 12.5x, D orig mag 200x).
With the goal of describing the pattern, distribution, severity, and risk factors of arteriosclerosis in HIV-positive patients, we studied the coronary arteries of a large autopsy population of HIV patients and compared our findings to controls.
Methods
Study Population:
We obtained the hearts of 66 deceased HIV-positive patients from the National Neurological AIDS Bank (NNAB), a tissue bank and clinical data repository of patients dying with advanced AIDS. Inclusion criteria for our sample were age <55 years, to limit age-related atherosclerosis as a confounding variable, and the availability of a heart suitable for examination and coronary sectioning. The past medical histories and cardiovascular risk factors of these patients were obtained from the NNAB database and supplemented with extensive chart reviews of available hospital and clinic notes and laboratory results. All patients enrolled in the NNAB signed an IRB approved informed consent, and the current study received a waiver of consent from the UCLA IRB. All study patients died between January 2001 and September 2006.
Control Population:
We also obtained the hearts of 19 HIV-negative controls, 4 chronically ill control patients from the NNAB study and 15 patients from randomly-selected, routine hospital autopsy cases. All control patients were <55 years of age and had hearts suitable for examination and coronary sectioning. Clinical data were collected via chart review where available. All control patients died between April 2002 and January 2007.
Tissue Handling and Processing:
All hearts were fixed in 10% buffered formalin for at least 24 hours. Following decalcification when gross calcification was present, we sampled the coronary arteries extensively, sectioning the entire left main (LM), the first two centimeters of the left anterior descending (LAD), the first two centimeters of the left circumflex (CIR), and the first three centimeters of the right coronary artery (RCA) with transverse cuts at 2-3mm increments. Each centimeter of artery, represented by 3-4 sections of 2-3mm thickness, was placed in a separate cassette for processing. All of these cassettes were processed routinely and embedded in paraffin. Each block was cut into histologic sections and stained with hematoxylin and eosin (H&E).
Histologic Analysis and Definitions:
Using light microscopy, each slide was examined simultaneously by three of the authors who were blinded to the HIV status of each case. The 3-4 sections on each slide were graded as a composite to represent the maximum extent and severity of disease in each 1cm segment of coronary artery. Each grade represented a consensus of all three individual graders. In all, over 1000 slides were reviewed.
First, we determined whether coronary arteries exhibited lesions consistent with cardiac transplant vasculopathy (Figure 1, Panel A, described above), typical atherosclerosis, adaptive intimal thickening, or normal histology. To this end, we judged whether a typical atherosclerotic plaque was present. Lipid-containing macrophages (foam cells), extracellular lipid collections, fibrous tissue, and calcification were criteria of an atherosclerotic plaque (Figure 1, Panel B).(19, 20) Sections without these characteristics but with mild, nonobstructive intimal thickening by connective tissue were termed adaptive intimal thickening (Figure 1, Panels C and D). This type of lesion is thought to be a common physiologic response to mechanical stresses secondary to variations in flow or wall tension.(21) This lesion is histologically similar to the fibromuscular proliferation seen in transplant coronary artery disease, but it does not obstruct the lumen and is easily distinguishable from it. Arteries with normal histology lack histologic evidence of any of these processes and resemble the area opposite the atherosclerotic plaque in Panel B of Figure 1.
Next, we graded each section in four basic categories in order to describe lesion severity, morphology, and composition. We recorded the percent luminal narrowing as a visual estimate of the percent cross-sectional area occupied by lesion from the internal elastic lamina inward. We also recorded the percent of vessel circumference involved and the percent of the lesion occupied by extracellular lipid collections (Figure 1, Panel B), also by visual estimation.
We graded the level of calcification in each section. Vessel sections with calcium deposits visible only at high power were termed grade 1 (Figure 2, Panel A). Those with diffuse, small calcium deposits visible at low power were grade 2 (Figure 2, Panel B). Large and coalescent calcium deposits visible at low power were termed grade 3 (Figure 2, Panel C). If no calcium deposits were visible even at high power, a grade of 0 was assigned. We also noted the following characteristics of complicated plaques when present—plaque fissure, plaque hemorrhage, and thrombus (Figure 3, Panel A)—characteristics associated with acute cardiovascular events, including sudden cardiac death.(20, 22) We also recorded any additional descriptive findings.
Figure 2:
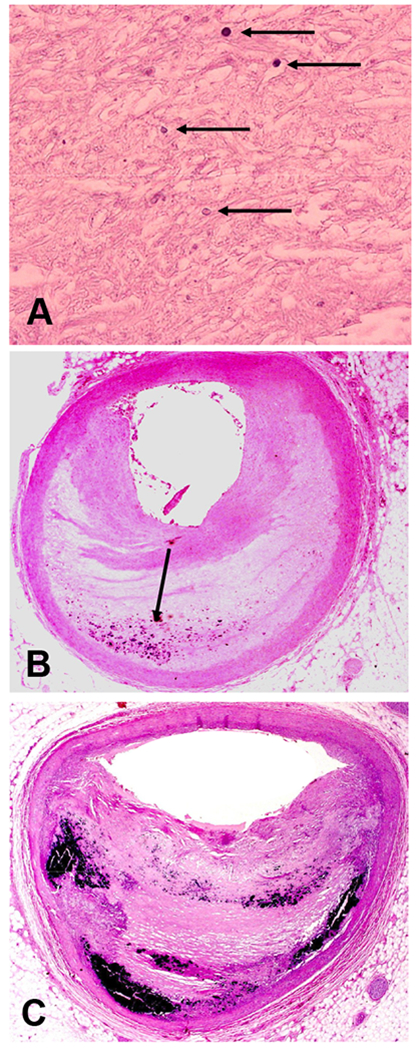
Quantification of calcification: A) Grade 1 calcification only seen at high magnification (arrows); B) Grade 2 calcification seen as small blue dots at low magnification (arrow); and C) Grade 3 calcification seen as prominent confluent blue regions at low magnification (all H&E, A orig mag 400x, B and C orig mag 12.5x)
Figure 3:
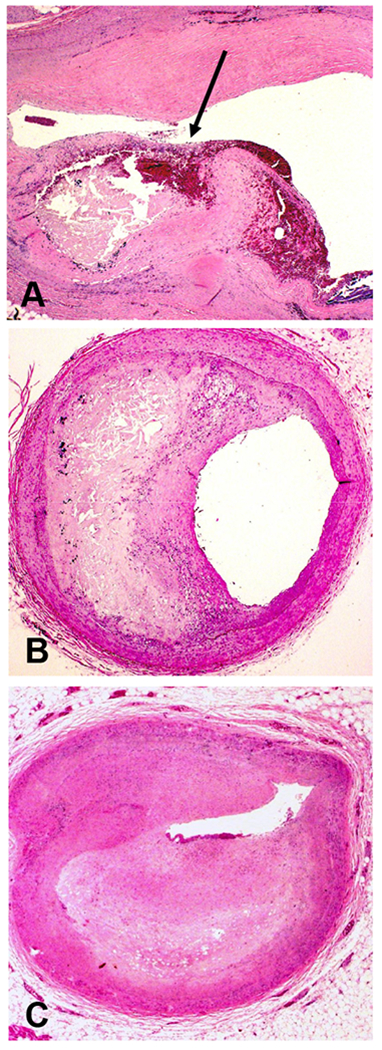
A) Complicated atherosclerotic plaque with plaque rupture (arrow). Note mural thrombus and plaque hemorrhage in region of plaque rupture; B) Atherosclerotic plaque with moderate, ≥50% luminal narrowing; and C) Atherosclerotic plaque with severe, ≥75% luminal narrowing (all H&E, A orig mag 20x, B and C orig mag 12.5x).
Statistical Analysis:
For the purpose of analysis, vessel sections were assigned to one of four categories based on luminal narrowing: Grade 1 (0-24%), Grade 2 (25-49%), Grade 3 (50-74%), and Grade 4 (75-100%). The use of this method for estimating the degree of coronary artery luminal stenosis has been validated by planimetry, showing both accuracy of visual inspection under magnification and agreement between independent observers.(23, 24) For the purposes of our analysis, grade 3 lesions resulting in ≥50% area stenosis (Figure 3, Panel B) were considered moderate, and grade 4 lesions resulting in ≥75% stenosis (Figure 3, Panel C) were considered severe.
We used Student’s t or Fisher exact tests to compare age and body mass index differences between HIV-positive patients and controls. When appropriate, we used a Satterthwaite approximation due to unequal variances. We used hierarchical linear or logistic random effects models to compare atherosclerotic narrowing ≥75%, calcium grade 2 or 3, and plaque lipid content (for plaques with stenosis ≥25%) across HIV-positive and control groups, controlling for age and sex differences. These models included random effects for subject and for artery within subject. Models were fit using WinBugs(25) and vague priors. We performed single predictor analyses adjusted for age and gender to determine factors associated with severe atherosclerosis and calcification in the HIV-positive population and then performed multivariate analyses to control for all significant univariate predictors.
Results:
Demographics
Demographic patient data are summarized in Table 1. The 66 HIV-positive patients had an average age of 43 years, while the 19 control patients averaged 39 years of age (p=0.06). Eighty-six percent of the HIV-positive patients were men compared to just 63% of the HIV-negative patients (p=0.02). In addition, a larger percentage of the HIV-positive patients used cocaine, smoked, and were hypertensive (blood pressure >140/90 or on antihypertensive drug therapy). Roughly the same proportion of HIV patients and controls were hyperlipidemic (total cholesterol > 200mg/dL; low density lipoproteins > 100mg/dL; or on drug therapy for hyperlipidemia). Fewer HIV patients were overweight (BMI ≥ 25) (p=0.06). None of these differences among important CAD risk factors achieved statistical significance except male sex. It should be noted that not all data were available for every patient.
Table 1.
Demographic characteristics
| HIV | Control | ||||
|---|---|---|---|---|---|
| Mean or % | N* | Mean or % | N* | p-value | |
| Age in years ± s.d. | 43.4 ± 0.8 | 66 | 39.0 ± 2.0 | 19 | 0.06 |
| Body Mass Index ± s.d. | 23.9 ± 0.7 | 40 | 30.2 ± 5.0 | 9 | 0.1 |
| Male sex | 86.4% | 66 | 63.2% | 19 | 0.02 |
| Alcohol/Drug use | 63.2% | 57 | 22.2% | 9 | 0.02 |
| Cocaine use | 29.8% | 57 | 22.2% | 9 | 1.0† |
| Smoking | 40.7% | 54 | 16.7% | 6 | 0.39 |
| Overweight | 30.6% | 49 | 66.7% | 9 | 0.06† |
| Diabetes Mellitus | 5.6% | 54 | 11.1% | 9 | 0.47† |
| Hypertension | 36.4% | 55 | 22.2% | 9 | 0.71† |
| Hyperlipidemia | 23.6% | 55 | 22.2% | 9 | 1.0† |
| Family History of CAD | 11.4% | 44 | 0.0% | 5 | 1.0† |
| Duration of HIV in years + s.d. | 9.3 + 5.8 | 61 | N/A | 0 | N/A |
| Duration of ART in years + s.d. | 4.6 + 3.5 | 50 | N/A | 0 | N/A |
| Last CD4 Count + s.d. | 128 + 155 | 59 | N/A | 0 | N/A |
Number of patients for whom data was available for the given demographic characteristic
Fisher’s Exact p-value; ART: antiretroviral therapy; CAD: coronary artery disease; CD4: T4 helper T-cell
All HIV-positive patients in this study died with advanced AIDS, and most were extensively HAART-treated. Seven of the control patients also died with chronic illnesses, such as amyotrophic lateral sclerosis, leukemia, or pancreatitis. The other 12 died suddenly out-of-hospital, most likely of cardiac arrhythmias not of ischemic etiology.
Extent of coronary atherosclerotic disease
Table 2 summarizes the histologic findings per 1cm coronary artery segment for HIV-positive and HIV-negative patients, unadjusted for age and sex differences. The obstructive lesions seen in these arteries were typical atherosclerotic plaques and were not consistent with the fibromuscular hyperplasia described in cardiac transplant vasculopathy and previously in HIV-positive patients.(16) Lesions consistent with cardiac transplant vasculopathy were not observed in these patients.
Table 2.
Results per 1cm coronary artery segment, unadjusted for age and sex differences
| HIV+ (n=66) | HIV− (n=19) | |
|---|---|---|
| Presence of an Atherosclerotic Plaque | 96% | 88% |
| Average Area Stenosis | 35% (± 6%) | 21% (± 7%) |
| Grade 1 Stenosis (0-24%) | 45% | 67% |
| Grade 2 Stenosis (25-49%) | 21% | 19% |
| Grade 3 Stenosis (50-74%) | 26% | 10% |
| Grade 4 Stenosis (75-100%) | 9% | 4% |
| Average Plaque Lipid Content | 25% (± 26%) | 11% (± 13%) |
| No Calcification | 14% | 45% |
| Grade 1 Calcification | 55% | 41% |
| Grade 2 Calcification | 9% | 5% |
| Grade 3 Calcification | 22% | 9% |
On average, atherosclerotic plaques obstructed 35±6% of the coronary artery lumen in HIV-positive patients, compared to 21±7% of the lumen in HIV-negative controls. In addition, the proportion of moderate or severe plaques (those resulting in luminal narrowing ≥50% or ≥75%, respectively) was more than two times greater in HIV-positive patients than HIV-negative controls.
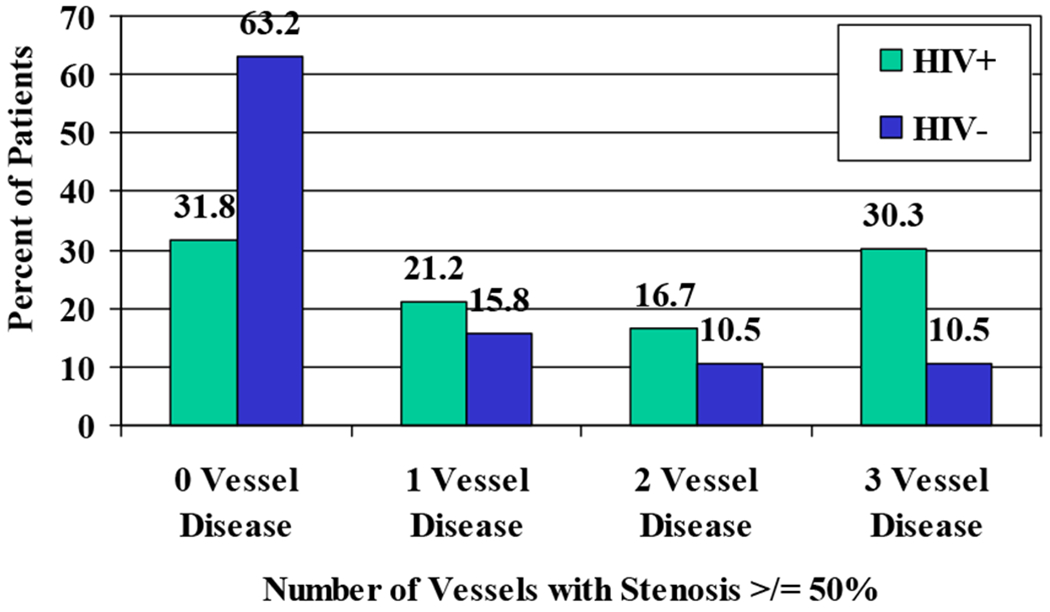
Sixty-eight percent of HIV-positive patients had luminal narrowing of ≥50% in at least one coronary artery, compared to 37% of HIV-negative controls (Figure 4). Thirty-five percent of HIV-positive patients had severe luminal narrowing ≥75% in at least one vessel, compared to 16% of HIV-negative controls. Five percent of HIV-positive patients had severe atherosclerotic narrowing ≥75% in all three major coronary arteries (Figure 5). Compared to historical controls of closely-matching age and sex(22) and controls in this study, a greater proportion of HIV-positive patients had narrowing ≥75% in one or multiple vessels (Table 3).
Figure 4:
Percent of all patients with moderate atherosclerotic disease: ≥50% vessel narrowing
Figure 5:
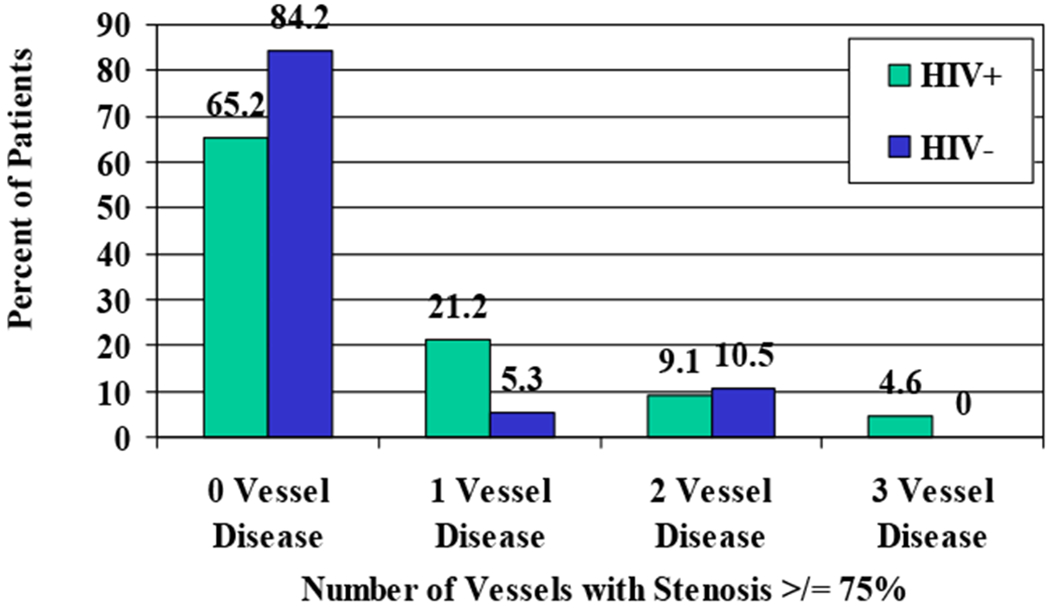
Percent of all patients with significant atherosclerotic disease: ≥75% vessel narrowing
Table 3.
Percent of patients with ≥75% vessel stenosis in one or more vessels: a comparison of the present findings to historical controls
| Present Study | Schmermund et al, 2001 | ||
|---|---|---|---|
| HIV+ (n=66) | HIV− (n=19) | Control (n=16) | |
| Age | 43 ± 6 | 39 ± 2 | 40 ± 6 |
| Male sex | 86% | 63% | 88% |
| BMI | 24 ± 5 | 30 ± 5 | 28 ± 9 |
| 0 vessel | 65% (43) | 84% (16) | 75% (12) |
| 1 vessel | 21% (14) | 5% (1) | 19% (3) |
| 2 vessel | 9% (6) | 11% (2) | 6% (1) |
| 3 vessel | 5% (3) | 0% (0) | 0% (0) |
Controlling for age and sex differences, we found that HIV-positive patients had three times greater odds of coronary artery luminal stenosis ≥75% than HIV-negative controls (OR=2.95, 1-sided p-value=0.03). As expected, older age and male sex were also significant predictors of stenosis ≥75% (1-sided p values <0.001) (Table 4).
Table 4.
Logistic hierarchical random effects model of stenosis ≥75% on age, sex, and HIV status
| Variable | OR | CI | 1-sided p-value |
|---|---|---|---|
| HIV+ | 2.95 | (0.94,9.39) | 0.03 |
| Age | 1.07 | (1.03,1.13) | <0.001 |
| Male Sex | 10.08 | (2.80,38.5) | <0.001 |
We observed complicated plaques featuring plaque hemorrhage, fissure, or thrombus(20) in 6% of coronary artery segments from HIV-positive patients and 0% of the segments from HIV-negative controls.
Composition and distribution of atherosclerotic plaques
As in the general population, most atherosclerotic plaques in both HIV-positive and HIV-negative patients were composed primarily of fibrous tissue.(26) Data concerning the lipid and calcium composition of the atherosclerotic plaques can be seen in Table 2. In unadjusted analysis, coronary artery segments in HIV-positive patients had greater atherosclerotic lipid content and larger, more frequent calcium deposits than those in HIV-negative controls.
Controlling for age and sex differences, we found that HIV seropositivity was associated with greater atherosclerotic plaque lipid content among plaques with at least 25% luminal stenosis (1-sided p=0.02). While neither age nor sex was associated with plaque lipid content, HIV seropositivity was associated with an 11% additive increase in the percent lipid composition of atherosclerotic plaques (Table 5).
Table 5.
Normal hierarchical random effects model of plaque lipid content on age, sex, and HIV status for plaques with stenosis ≥25%
| Variable | Plaque Lipid Content | CI | 1-sided p-value |
|---|---|---|---|
| HIV+ | 11% additive increase | (0,23) | 0.02 |
| Age | 0% additive increase/year | (0,1) | 0.89 |
| Male Sex | 6% additive increase | (−6,19) | 0.16 |
Using a similar logistic random effects model, we found that HIV seropositivity also tended to be associated with high, grade 2 or 3, atherosclerotic calcification (OR=2.44; 1-sided p=0.08). However, neither HIV-status, nor age, nor sex was a statistically significant predictor of high levels of coronary calcification in this analysis.
Distribution of the lesions:
Atherosclerotic plaques resulted in marginally greater coronary artery stenosis in more proximal segments, both in HIV-positive and HIV-negative patients, except in the right coronary artery, where plaques were more evenly distributed (data not shown). We rarely observed gross plaques distal to the areas sampled for microscopy. Atherosclerotic plaques were nearly always eccentric rather than concentric. On average, 77% of the vessel circumference was involved with plaque in HIV-positive patients, compared with 73% of the vessel circumference in HIV-negative controls. We did not observe intramyocardial artery involvement seen in cardiac transplant vasculopathy.
General and HIV-specific risk factors for coronary atherosclerosis
In the HIV-positive population, known CAD risk factors such as older age, male sex, smoking, hypertension and diabetes as well as white ethnicity were related to moderate or severe atherosclerotic luminal narrowing (≥50%) and coronary calcification (grade 2 or 3). HIV-specific factors including higher last CD4 count, higher nadir CD4 count, and lower last viral load were also associated with these markers of atherosclerotic disease. However, after controlling for all significant univariate factors, only older age and hypertension predicted greater luminal stenosis. African-American ethnicity was protective for coronary calcification. Neither the duration of HIV infection nor the duration of antiretroviral therapy or protease inhibitor therapy predicted more significant atherosclerotic disease. Markers of immunologic status and HIV progression, CD4 count and viral load, were also not associated with severe atherosclerotic disease in multivariate analysis.
Dystrophic Calcification
We observed two patterns of dystrophic vascular calcification not previously described in the medical literature—calcification limited to the internal elastic lamina (IEL) of coronary arteries and calcium oxalate crystal deposition in coronary artery atherosclerotic plaques. Nearly all the patients in whom these calcification patterns were observed, both AIDS patients and older controls, suffered from chronic illnesses, such as advanced AIDS, amyotrophic lateral sclerosis, or metastatic cancer. These findings are described and discussed in detail elsewhere (in press in Modern Pathology and Cardiovascular Pathology, respectively).
Discussion:
Extent of coronary atherosclerotic disease
In this study, the largest morphologic study of coronary arteries in HIV-positive patients to date, we confirm a high burden of lesions with the typical morphology of atherosclerotic plaques. Thirty-five percent of these HIV-positive patients had ≥75% narrowing in at least one coronary artery. In addition, a significant proportion had multivessel disease, and 6% of coronary artery segments were involved with complicated plaques. These markers of disease are important reference points in other histologic studies.(22, 24, 27) Compared to historical controls, these findings indicate a greater than usual burden of atherosclerosis in a patient population with an average age of 43 years.(22)
Compared to the relatively small group of controls available for study, the HIV-positive patients we examined had three times greater odds of coronary artery stenosis ≥75%, controlling for age and sex differences. While larger and better-matched control groups are needed before final conclusions can be drawn, these data suggest the burden of coronary artery disease is increased in HIV-positive patients. These histologic findings support the conclusions of previous studies suggesting an increased risk of myocardial infarction,(28) and subclinical atheroslcerosis in HIV-infected patients.(3) The present study adds histologic substantiation and description of the arterial lesions to this clinical evidence.
Composition and distribution of atherosclerotic plaques
While previous studies have gauged the risk of coronary events or estimated the amount of preclinical disease in this population, they have not described the composition, pattern, and distribution of the lesions. Some have suggested HIV-associated arteriosclerosis has a histologic appearance similar to cardiac transplant vasculopathy;(16) if so, it might be explained by an analogous, immune-mediated mechanism.(29)
Based on clinical experience and published reports, however, the pattern of disease and composition of the lesions we observed in these patients is not similar to transplant vasculopathy but is, rather, consistent with atherosclerosis seen in the general population. Instead of concentric fibromuscular hyperplasia of the intima seen in transplant vasculopathy, atherosclerotic plaques in both HIV-positive and control patients were eccentric lesions composed of fibrous tissue, lipid, and calcium like those described in large autopsy studies of atherosclerosis in the general population.(20) Fibromuscular hyperplasia in transplant vasculopathy generally affects both the proximal and distal portions of coronary arteries. However, in this study, significant atherosclerotic lesions were concentrated in the proximal segments of the left anterior descending and left circumflex arteries and were more uniform in the right coronary artery in both HIV-positive and HIV-negative patients. These findings are consistent with previous studies in HIV-negative individuals.(30, 31)
Atherosclerotic plaques in HIV-positive patients were not only larger in overall size than those in HIV-negative patients, they were also composed of significantly higher proportions of extracellular lipid. In their study of coronary atherosclerosis in sudden coronary death, Schmermund et al found that greater plaque area and lipid core area were independently associated with plaque rupture leading to sudden death.(22) Stable plaques, without histologic signs of rupture or instability, were smaller and composed of less lipid. Previous reports have also asserted that large plaques are those most at risk of rupture(26, 32) and that large lipid collections contribute to plaque instability.(33, 34) If true, these findings suggest that, though atherosclerotic plaques in HIV-positive patients have the same general pattern and composition as those in the general population, these plaques may be more prone to developing life-threatening complications like hemorrhage, rupture, and thrombus than those in HIV-negative patients of the same age and sex.
Coronary atherosclerosis in HIV-positive patients is also frequently associated with heavy calcification. Twenty-two percent of coronary artery segments from HIV-positive patients exhibited the highest, grade 3 calcification, and HIV seropositivity was positively associated with significant grade 2 or 3 calcification. Though this association was not significant when controlling for age and sex, it is possible comparing larger HIV-positive and control populations might yield statistically significant differences. In any case, a significant number of HIV-positive patients exhibited high-grade vascular calcification, a characteristic of advanced atherosclerotic lesions.(20) The clinical significance of atherosclerotic calcification is unclear. Some studies suggest calcific deposits are a feature of plaques that rupture, while others believe they actually increase plaque stability.(35) In the study by Schmermund et al, plaque calcification and calcified plaque area were both positively associated with plaque rupture in univariate analysis but not in multivariate analysis. Though calcification was a frequent feature of plaque rupture, perhaps reflecting the larger size of such plaques, lipid content and plaque size were better predictors of plaque rupture.(22)
It is interesting to note that several studies have measured coronary artery calcification (CAC) in HIV-positive patients using electron beam computed tomography (EBCT), and none have found impressive levels of calcification in such patients as compared to controls.(36–39) While EBCT for detecting CAC has been validated histologically in the general population, it has not been compared to histologic findings in the HIV-positive population.
General and HIV-specific risk factors for coronary atherosclerosis
A central question in the study of atherosclerosis in HIV is whether HIV infection or HAART contribute to atherosclerotic disease. In this study, we found no evidence that any HIV-specific factor other than HIV seropositivity is associated with histologic markers of severe atherosclerosis. Neither duration of infection, duration of HAART, nor protease inhibitor exposure predicted more severe disease. It is important to note, however, that our study population was relatively small (n=66). Most patients were exposed to antiretroviral drugs from all major classes. This rather homogenous treatment history and small sample size may have made it difficult to uncover subtle differences in treatment-related risk.
Dystrophic Vascular Calcification and Mechanisms of Disease
HIV-positive patients, particularly those heavily treated with HAART, frequently suffer from metabolic disorders associated with dystrophic vascular calcification, including osteopenia/osteoporosis and secondary hyperparathyroidism.(40, 41) These conditions upset the delicate balance of inhibitory and stimulatory factors which dictates the tightly-regulated, active process of vascular calcification.(35) Additionally, protease inhibitors used in HIV therapy have been shown to alter genes involved in calcium metabolism in vitro.(42) It is tempting to speculate that metabolic derangements in HIV patients predispose them to atherosclerosis as well as the unusual forms of dystrophic calcification we observed in this study (in press in Modern Pathology and Cardiovascular Pathology). Because these patterns of calcification were present in study patients and older control patients with advanced chronic diseases, it is also tempting to speculate that immune activation, chronic inflammation, or aging may contribute to these findings.
Limitations and Future Directions
While our study (n=66) is significantly larger than any previous histologic study (n=15) on this subject,(15, 16) it is small by comparison to the multi-center epidemiologic studies concerning coronary artery disease in HIV-infected patients. This difference is consistent with natural limitations in autopsy study enrollment.
This study examined a highly specialized population of HIV-infected patients with advanced AIDS. It is not clear whether HIV-positive patients with functional immune systems would have similar atherosclerotic disease burdens.
Though there was no statistically significant difference in rates of smoking, hypertension, hyperlipidemia, cocaine use, and other CAD risk factors between these two groups, the subpopulations were at times quite small. It is possible larger studies would demonstrate significant differences in the prevalence of these factors, necessitating statistical adjustment to control for these differences.
No perfusion-fixed specimens were available for this study. We estimated luminal narrowing without the advantage of this tool. However, all specimens from both HIV-positive and HIV-negative patients were graded in a blinded process using identical criteria. Any inaccuracies resulting from a lack of perfusion-fixed vessels should not have introduced bias into our findings.
Conclusions
This study demonstrates that young to middle-aged HIV-positive patients have a burden of CAD that results in luminal narrowing, heavy calcification, and high plaque lipid content, characteristics associated with plaque rupture and sudden cardiac death. The evidence also suggests their odds of having severe disease may be increased compared to HIV-negative controls.
Based on published reports and clinical experience, the pattern of disease and atherosclerotic plaque composition in HIV-positive patients is similar to that observed in the HIV-negative population. Previously unreported patterns of dystrophic vascular calcification were seen in HIV-positive patients as well as older, HIV-negative controls with chronic illnesses.
Future studies are needed to elucidate the pathogenesis of atherosclerosis and dystrophic vascular calcification in patients with HIV, chronic disease, and older age.
Acknowledgements:
Special thanks to David Jones and Kaz Ando of the National Neurological AIDS Bank for facilitating access to tissue samples and patient histories.
Special thanks to Longsheng Hong for her histology laboratory expertise.
Funding Sources:
This project was made possible by generous funding contributions from:
The National Neurological AIDS Bank (EJS)
The Piansky Family Trust (MCF)
NIH K24 AI56933 (JSC)
UCLA CFAR grant AI28697 (REW)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
None
Contributor Information
Robert G. Micheletti, University of California, Los Angeles.
Gregory A. Fishbein, University of California, Los Angeles.
Michael C. Fishbein, University of California, Los Angeles.
Elyse J. Singer, University of California, Los Angeles.
Robert E. Weiss, University of California, Los Angeles.
Robin A. Jeffries, Public Health—Biostatistics, University of California, Los Angeles.
Judith S. Currier, University of California, Los Angeles.
References
- 1.Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 2.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, Thiebaut R, De Wit S, Kirk O, Fontas E, Law MG, Phillips A, Lundgren JD. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–35. [DOI] [PubMed] [Google Scholar]
- 3.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, Waters DD. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004; 109:1603–8. [DOI] [PubMed] [Google Scholar]
- 4.Stein JH, Klein MA, Bellehumeur JL, McBride PE, Wiebe DA, Otvos JD, Sosman JM. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation 2001; 104:257–62. [DOI] [PubMed] [Google Scholar]
- 5.van Wijk JP, de Koning EJ, Cabezas MC, Joven J, op’t Roodt J, Rabelink TJ, Hoepelman AM. Functional and structural markers of atherosclerosis in human immunodeficiency virus-infected patients. J Am Coll Cardiol 2006; 47:1117–23. [DOI] [PubMed] [Google Scholar]
- 6.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. Aids 2003; 17:2479–86. [DOI] [PubMed] [Google Scholar]
- 7.Mulligan K, Grunfeld C, Tai VW, Algren H, Pang M, Chernoff DN, Lo JC, Schambelan M. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr 2000; 23:35–43. [DOI] [PubMed] [Google Scholar]
- 8.Mujawar Z, Rose H, Morrow MP, Pushkarsky T, Dubrovsky L, Mukhamedova N, Fu Y, Dart A, Orenstein JM, Bobryshev YV, Bukrinsky M, Sviridov D. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol 2006; 4:e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazenberg MD, Stuart JW, Otto SA, Borleffs JC, Boucher CA, de Boer RJ, Miedema F, Hamann D. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART). Blood 2000; 95:249–55. [PubMed] [Google Scholar]
- 10.Wolf K, Tsakiris DA, Weber R, Erb P, Battegay M. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis 2002; 185:456–62. [DOI] [PubMed] [Google Scholar]
- 11.Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 1999; 145:33–43. [DOI] [PubMed] [Google Scholar]
- 12.Libby P Inflammation in atherosclerosis. Nature 2002; 420:868–74. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003; 107:363–9. [DOI] [PubMed] [Google Scholar]
- 14.Ross R Atherosclerosis--an inflammatory disease. N Engl J Med 1999; 340:115–26. [DOI] [PubMed] [Google Scholar]
- 15.Paton P, Tabib A, Loire R, Tete R. Coronary artery lesions and human immunodeficiency virus infection. Res Virol 1993; 144:225–31. [DOI] [PubMed] [Google Scholar]
- 16.Tabib A, Leroux C, Mornex JF, Loire R. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis 2000; 11:41–6. [DOI] [PubMed] [Google Scholar]
- 17.Ventura HO, Mehra MR, Smart FW, Stapleton DD. Cardiac allograft vasculopathy: current concepts. Am Heart J 1995; 129:791–9. [DOI] [PubMed] [Google Scholar]
- 18.Waller J, Brook NR, Nicholson ML. Cardiac allograft vasculopathy: current concepts and treatment. Transpl Int 2003; 16:367–75. [DOI] [PubMed] [Google Scholar]
- 19.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994; 89:2462–78. [DOI] [PubMed] [Google Scholar]
- 20.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 1995; 15:1512–31. [DOI] [PubMed] [Google Scholar]
- 21.Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull W Jr, Richardson M, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1992; 85:391–405. [DOI] [PubMed] [Google Scholar]
- 22.Schmermund A, Schwartz RS, Adamzik M, Sangiorgi G, Pfeifer EA, Rumberger JA, Burke AP, Farb A, Virmani R. Coronary atherosclerosis in unheralded sudden coronary death under age 50: histo-pathologic comparison with ‘healthy’ subjects dying out of hospital. Atherosclerosis 2001; 155:499–508. [DOI] [PubMed] [Google Scholar]
- 23.Isner JM, Wu M, Virmani R, Jones AA, Roberts WC. Comparison of degrees of coronary arterial luminal narrowing determined by visual inspection of histologic sections under magnification among three independent observers and comparison to that obtained by video planimetry: an analysis of 559 five-millimeter segments of 61 coronary arteries from eleven patients. Lab Invest 1980; 42:566–70. [PubMed] [Google Scholar]
- 24.Mizgala HF, Gray LH, Ferris JA, Bociek V, Allard P, Davies C. Coronary artery luminal narrowing in the young with sudden unexpected death: a coroner’s autopsy study in 350 subjects age 40 years and under. Can J Cardiol 1993; 9:33–40. [PubMed] [Google Scholar]
- 25.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS--a Bayesian modeling framework: concepts, structure, and extensibility. Statistics and Computing 2000; 10:325–37. [Google Scholar]
- 26.Falk E Morphologic features of unstable atherothrombotic plaques underlying acute coronary syndromes. Am J Cardiol 1989; 63:114E–20E. [DOI] [PubMed] [Google Scholar]
- 27.Joseph A, Ackerman D, Talley JD, Johnstone J, Kupersmith J. Manifestations of coronary atherosclerosis in young trauma victims--an autopsy study. J Am Coll Cardiol 1993; 22:459–67. [DOI] [PubMed] [Google Scholar]
- 28.Klein D, Hurley LB, Quesenberry CP Jr, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr 2002; 30:471–7. [DOI] [PubMed] [Google Scholar]
- 29.Hsue PY, Waters DD. What a cardiologist needs to know about patients with human immunodeficiency virus infection. Circulation 2005; 112:3947–57. [DOI] [PubMed] [Google Scholar]
- 30.Hochman JS, Phillips WJ, Ruggieri D, Ryan SF. The distribution of atherosclerotic lesions in the coronary arterial tree: relation to cardiac risk factors. Am Heart J 1988; 116:1217–22. [DOI] [PubMed] [Google Scholar]
- 31.Wang JC, Normand SL, Mauri L, Kuntz RE. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation 2004; 110:278–84. [DOI] [PubMed] [Google Scholar]
- 32.Fishbein MC, Siegel RJ. How big are coronary atherosclerotic plaques that rupture? Circulation 1996; 94:2662–6. [DOI] [PubMed] [Google Scholar]
- 33.Davies MJ. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation 1996; 94:2013–20. [DOI] [PubMed] [Google Scholar]
- 34.Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J 1993; 69:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 2006; 99:1044–59. [DOI] [PubMed] [Google Scholar]
- 36.Acevedo M, Sprecher DL, Calabrese L, Pearce GL, Coyner DL, Halliburton SS, White RD, Sykora E, Kondos GT, Hoff JA. Pilot study of coronary atherosclerotic risk and plaque burden in HIV patients: ‘a call for cardiovascular prevention’. Atherosclerosis 2002; 163:349–54. [DOI] [PubMed] [Google Scholar]
- 37.Lai S, Lai H, Celentano DD, Vlahov D, Ren S, Margolick J, Lima JA, Bartlett JG. Factors associated with accelerated atherosclerosis in HIV-1-infected persons treated with protease inhibitors. AIDS Patient Care STDS 2003; 17:211–9. [DOI] [PubMed] [Google Scholar]
- 38.Robinson FP, Hoff JA, Kondos GT. Coronary artery calcium in HIV-infected men treated with highly active antiretroviral therapy. J Cardiovasc Nurs 2005; 20:149–54. [DOI] [PubMed] [Google Scholar]
- 39.Talwani R, Falusi OM, Mendes de Leon CF, Nerad JL, Rich S, Proia LA, Sha BE, Smith KY, Kessler HA. Electron beam computed tomography for assessment of coronary artery disease in HIV-infected men receiving antiretroviral therapy. J Acquir Immune Defic Syndr 2002; 30:191–5. [DOI] [PubMed] [Google Scholar]
- 40.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. Aids 2006; 20:2165–74. [DOI] [PubMed] [Google Scholar]
- 41.Seminari E, Castagna A, Soldarini A, Galli L, Fusetti G, Dorigatti F, Hasson H, Danise A, Guffanti M, Lazzarin A, Rubinacci A. Osteoprotegerin and bone turnover markers in heavily pretreated HIV-infected patients. HIV Med 2005; 6:145–50. [DOI] [PubMed] [Google Scholar]
- 42.Malizia AP, Cotter E, Chew N, Powderly WG, Doran PP. HIV protease inhibitors selectively induce gene expression alterations associated with reduced calcium deposition in primary human osteoblasts. AIDS Res Hum Retroviruses 2007; 23:243–50. [DOI] [PubMed] [Google Scholar]


