Figure 2. SPY Physically Interacts with and O-Fucosylates PRR5.
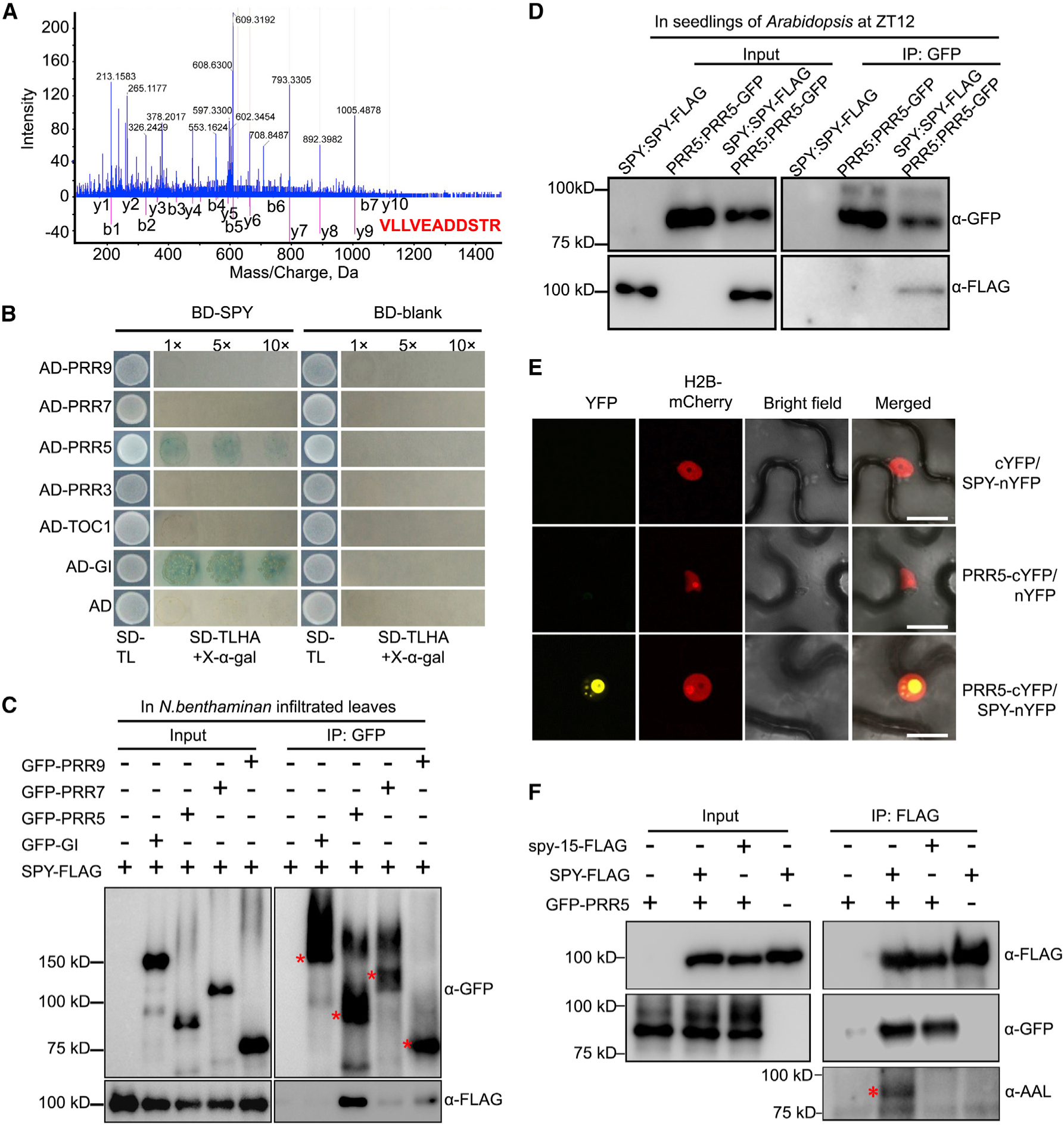
(A) Peptides of PRR5 protein were identified by affinity purification followed by mass spectrometry. Representative tandem mass spectrometry spectrum of the peptide corresponding to PRR5 protein (VLLVEADDSTR) is shown.
(B) Yeast two-hybrid assay showing that PRR5 interacts with SPY. Yeast clones were grown on synthetic dropout (SD) medium devoid of tryptophan and leucine (SD-TL) for initial screening (first column), before being transferred to SD medium devoid of tryptophan, leucine, histidine, and adenine, plus X-gal (SD-TLHA + 40 μg/ml X-gal) with dilution factor as indicated (second column).
(C) Co-immunoprecipitation analysis of SPY-FLAG with GFP-tagged circadian components. GFP-Trap beads were used to precipitate protein complexes extracted from co-infiltrated leaves of N. benthamiana. Red asterisks indicate the major bands of the correspondingly indicated proteins.
(D) Co-immunoprecipitation analysis of SPY with PRR5 in Arabidopsis. Both SPY-FLAG and PRR5-GFP were driven by their respective promoters. SPY-FLAG and PRR5-GFP alone were used as negative controls.
(E) SPY physically interacts with PRR5 in the nucleus in BiFC assay. H2B-mCherry was used as nuclear marker. Scale bars, 10 μm.
(F) PRR5 is O-fucosylated by SPY. Proteins were extracted from the co-infiltrated N. benthamiana leaves with indicated combinations for coimmunoprecipitation, with anti-FLAG antibody-conjugated beads. The antibodies used for detecting the respective protein are listed. The asterisk indicates the specific SPY-O-fucosylated PRR5 protein band. The representative data are from three individual biological replicates with similar results.
