Figure 3. Defining the Domain Requirement Bridging the Interaction between SPY and PRR5.
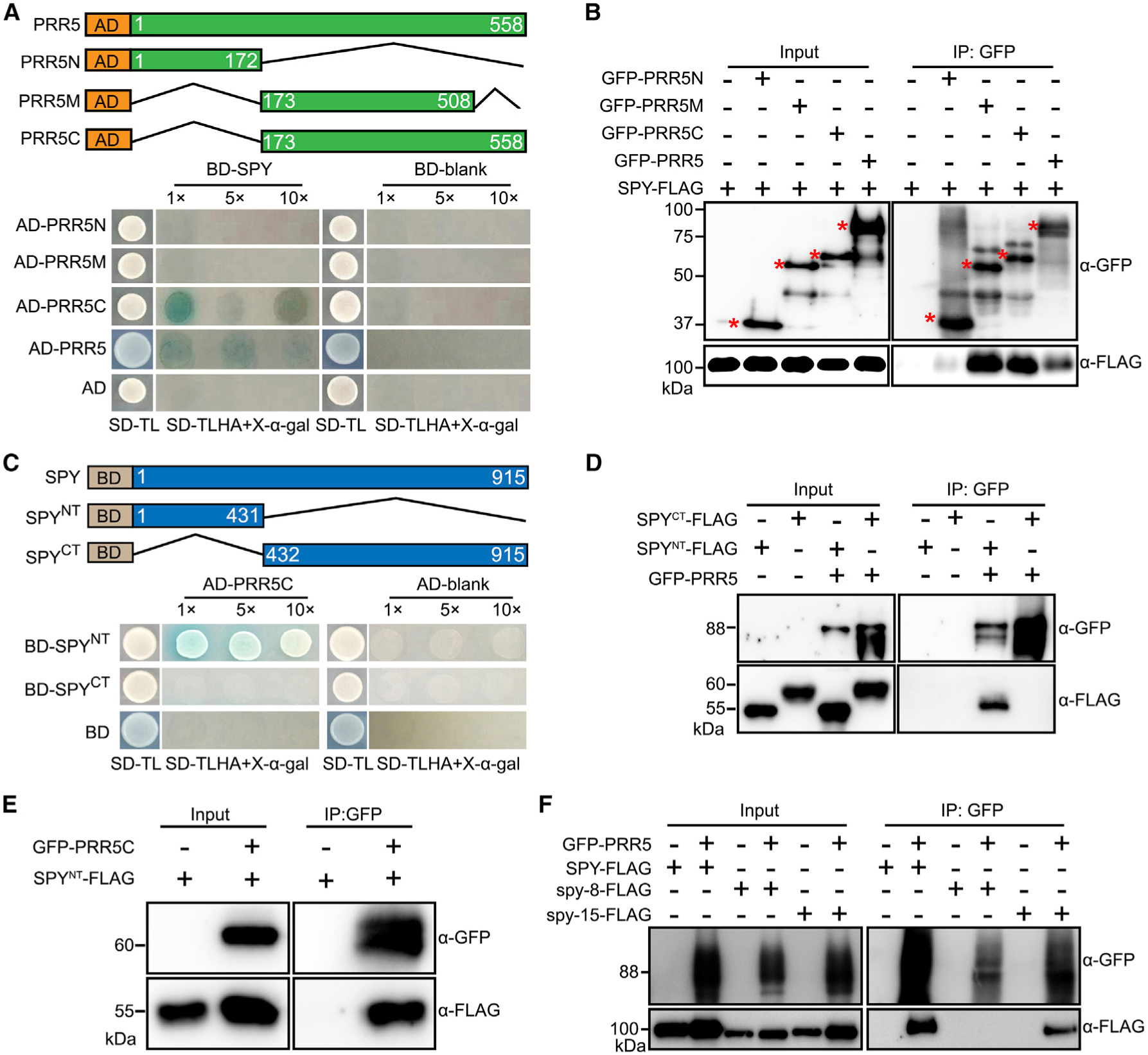
(A) Yeast two-hybrid assays showing that the C terminus of PRR5 is required for interacting with SPY. (Top) Schematic diagram of the constructs used for yeast two-hybrid assays. Numbers indicate the amino acid position. (Bottom) Yeast clones were grown on synthetic dropout (SD) medium devoid of tryptophan and leucine (SD-TL) for initial screening (first column), before being transferred to SD medium devoid of tryptophan, leucine, histidine, and adenine, plus X-α-gal (SD-TLHA + 40 μg/ml X-α-gal) with dilution factor as indicated (second column).
(B) Co-immunoprecipitation assay showing SPY interacts with PRR5 C terminus in vivo. SPY-FLAG was transiently expressed alone or co-expressed with PRR5N, PRR5M, and PRR5C. The input and IP samples were analyzed by immunoblotting using FLAG antibody and GFP antibodies, respectively.
(C) Yeast two-hybrid assays showing that SPY TPR region is essential for mediating the interaction between SPY and PRR5.
(D) Co-immunoprecipitation assay showing SPY TPR region is necessary and sufficient to interact with PRR5 in vivo. Assay performed as in B.
(E) Co-immunoprecipitation assay showing PRR5 C terminus is able to interact with SPY TPR region in vivo.
(F) Co-immunoprecipitation assay showing TPR domain mutation of SPY abolishes its interaction with PRR5, but catalytic domain mutation of SPY can interact with PRR5. The spy-8-FLAG contains a truncated TPR domain with from M354 to Q376, while spy-15-FLAG has a point mutation, E567K, which is considered as an essential amino acid residues for its catalytic activity.
