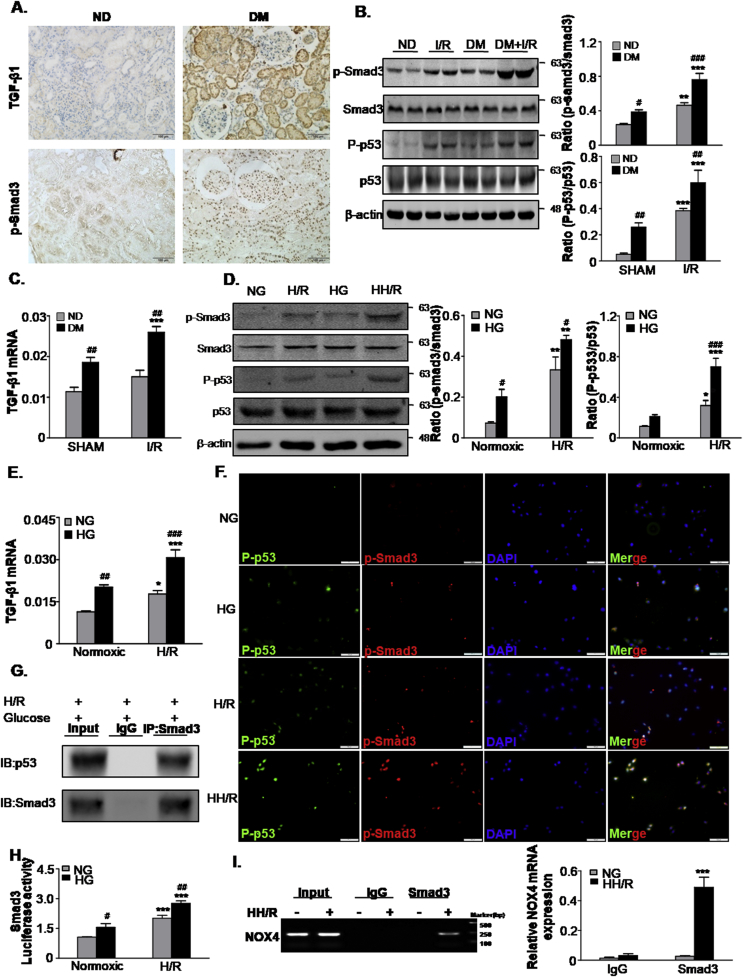
Fig. 5.
TGF-β/Smad3 levels increased in human diabetic kidneys, STZ-induced diabetic mice and high glucose-conditioned TECs.
A. Immunohistochemistry staining of TGF-β1 and phosphorylated Smad3 in human normal and diabetic patient tissues. Scale bars = 100 μm; B. Western blot analysis showed protein expression of p-Smad3, Smad3, P-p53 and p53 in mice; C. mRNA level of TGF-β1 in mice; D. Western blot analysis showing protein expression of p-Smad3, Smad3, P-p53 and p53 in TECs; E. mRNA levels of TGF-β1 in TECs; F. Double-immunofluorescence showing representative colocalization of P-p53 with p-Smad3 in TECs. Scale bars = 100 μm; G. Co-IP assay detected an interaction of Smad3 with p53; H. Luciferase reporter assay; I. Binding of Smad3 to NOX4 by ChIP assay. Data represent the mean ± S.E.M. for 6–8 mice in vivo and at least 3–4 independent experiments in vitro. *P < 0.05, **P < 0.01, ***P < 0.001 compared to SHAM group or Normoxic group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to ND group or NG group. ND: non-diabetic mice; DM: STZ-induced diabetic mice; I/R and DM + I/R: non-diabetic mice and STZ-induced diabetic mice were subject to ischemia reperfusion injury; NG: 5.5 mM glucose plus mannitol 24.5 mM mannitol; HG: high glucose; H/R: hypoxia/reoxygenation; HH/R: H/R under HG condition.