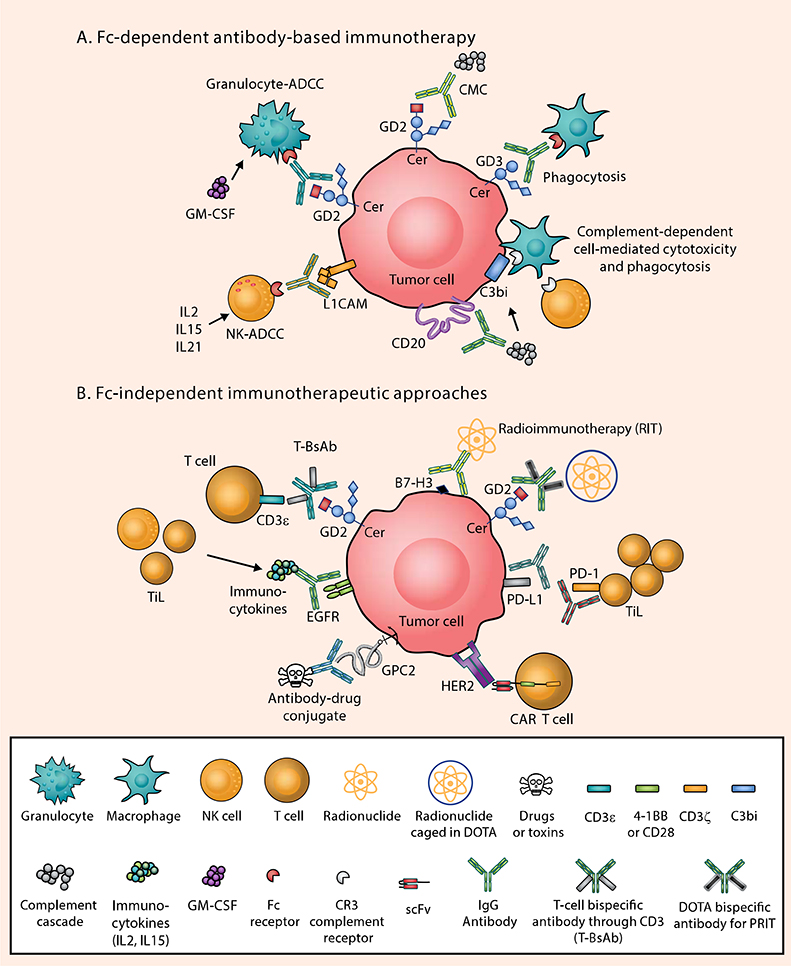
Figure 1. Antibody-based Immunotherapy in Pediatric Solid Tumors: Fc-dependent and Fc-independent Mechanisms.
The low mutational burden in pediatric solid tumors, the immaturity of the immune system in children, downregulation of tumor HLA molecules, and the use of intensive chemotherapy all combine to make the tumor microenvironment of pediatric solid tumors poorly immunogenic (8). Examples of surface antigen–antibody pairs include: B7-H3 [Omburtamab, 8H9], CD20 [Rituximab], EGFR [cetuximab], GD2 [Naxitamab or Dinutuximab], GD3 [R24], HER2 [trastuzumab], PD-1 [Pembrolizumab or Nivolumab], PD-L1 [Atezolizumab]. A) In the presence of IgG monoclonal antibodies targeting one of these antigens, tumor cells succumb to Fc-dependent cytotoxic killing: antibody-dependent NK cell–mediated cytotoxicity (NK-ADCC); granulocyte-mediated ADCC (granulocyte-ADCC); complement-mediated cytotoxicity (CMC) by binding to C1q (thereby activating the complement cascade); antibody-dependent macrophage-mediated phagocytosis; and CR3-dependent cell-mediated cytotoxicity through recognition of complement breakdown products (e.g. C3bi). B) Through genetic engineering, IgG antibodies can now be reformatted to engage T cells, which have no Fc receptor functions, by exploiting chimeric antigen receptors (CARs) or bispecific antibodies (anti-GD2 × anti-CD3), where polyclonal T cells can be driven into cold tumors as tumor-infiltrating lymphocytes (TILs) to initiate the tumor killing process. Multistep pre-targeted radioimmunotherapy (PRIT) and external-beam radiation therapy can also be utilized to increase neoantigen presentation and TIL infiltration. Immune checkpoint inhibitors (ICIs) as a monotherapy have been largely unsuccessful in pediatric solid tumors due to the paucity of neoepitopes and low number of TILs, but combinatorial approaches with radiotherapy, bispecifics, or CAR T cells that can attract TILs are promising for enhanced functioning of ICIs. Cytokines such as IL2, IL15, and IL21 can also combat the “cold” phenotype by attracting T cells and NK cells to the tumor. CR3, complement receptor 3; EGFR, epidermal growth factor receptor; GD2, disialoganglioside GD2; GD3, disialoganglioside GD3; GPC2, glypican-2; HER2, human epidermal growth factor receptor 2; L1CAM, L1 cell-adhesion molecule.