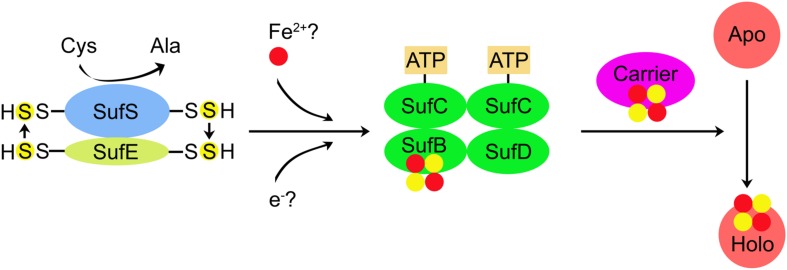
FIGURE 2.
A proposed model for the assembly of Fe–S clusters by SUF system in cyanobacteria. Fe–S cluster biogenesis is initiated by SufS (cysteine desulfurase), which converts cysteine (Cys) to alanine (Ala). Sulfane (S0) is transferred from SufS to SufE (sulfur transferase) and then to SufB of SufBC2D scaffold complex and bound as a persulfide (S2–). Putative Fe and electron (for reduction S0 to S2–) donors are still unknown. SufC has an ATPase activity, thus coupling ATP hydrolysis with the formation of Fe–S clusters. Subsequently, the newly assembled Fe–S cluster is transferred to the carrier protein, which delivers the Fe–S cluster to apo-protein (Apo) and further converts Apo to holo-protein (Holo). SufA and Slr0067 (Synechocystis 6803) may function as the carrier proteins.