Abstract
Lactic acid bacteria are the main bacterial group associated to meat spoilage. Herbal essential oils are promising alternatives that can be used to retard lactic acid bacterial growth and extend shelf life of meat products. In this study, the influence of oregano and rosemary essential oils on the growth of lactic acid bacteria and the physicochemical properties of refrigerated vacuum-packed Tuscan sausage was evaluated. In addition to the control (without the addition of oil), the sausage samples were separately treated with different concentrations of each essential oil (0.05 wt%, 0.1 wt%, 0.2 wt%, and 0.4 wt%). The shelf life was evaluated as the time to the lactic acid bacteria population to reach the levels of 106 and 107 CFU/g. After the addition of 0.05 wt% and 0.1 wt% of essential oil to the sausage, the rosemary essential oil provided a higher extension of the shelf life of the sausages (approximately 3 and 5 days, respectively) than the oregano essential oil (approximately 1 and 3 days, respectively). After adding 0.2 wt% and 0.4 wt% of essential oil, the oregano essential oil resulted in a larger increase of the shelf life of the samples (about 8 and 14 days, respectively) when compared with the rosemary essential oil (about 7 to 11 days, respectively). All the treatments slowed the growth of the lactic acid bacteria but they did not change the maximum bacterial population. New empirical models that relate the shelf life of the sausage and the maximum specific growth rate of the lactic acid bacteria with the oil concentration were obtained. These results can be applied to model the influence of essential oils on the shelf life of different meat products.
Keywords: Meat products, Shelf life, Oregano essential oil, Rosemary essential oil, Spoilage
Introduction
Brazil is one of the main meat producers and exporters in the world [1, 2]. Of the different meat products, fresh sausages are among the most commonly consumed processed meat products in the world [3, 4]. Lactic acid bacteria (LAB) are the main bacterial group associated with the spoilage of meat and meat products, especially in vacuum-packed products like Tuscan sausage [5–7]. The growth of LAB is favored by the combination of microaerophilic conditions in the presence of salt (NaCl), sodium nitrite (NaNO2), sodium nitrate (NaNO3), and the high water activity found in such products [8]. These products are usually stored at 4 °C before consumption and antimicrobial or antioxidant preservatives are employed to extend their shelf lives [9].
The shelf life of fresh sausages is not easily estimated because the deterioration of these products is a complex process which may result in the change of different sensory quality attributes (color, odor, flavor, and taste) [9]. The most important factor influencing these changes is the microbial growth [10]. As a result, the amount of natural LAB can be used to evaluate the shelf life of not only fresh sausages but also of vacuum-packed meat products in general [11]. The acceptable quality limits of LAB counts found in the literature for cooked meat products range from 6 to 8 log CFU/g [12–14]. One of the main techniques used to extend the shelf life of fresh sausages involves the use of chemical preservatives, such as lactate and acetate [12, 15]. Chemical or commercial synthetic preservatives are therefore widely employed in these products [16].
The use of natural antimicrobial components as preservative agents has increased due to the consumer demand for more natural food products [17, 18]. In this context, essential oils (EOs) are complex, natural, and volatile compounds. They can be found in aromatic plants as secondary metabolites and have a natural and inherently strong odor. EOs are made up of 20 to 60 components, and usually two or three major components in relatively high concentrations are responsible for the bioactivity [19]. Oregano essential oil (Origanum vulgare) (OEO) and rosemary essential oil (Rosmarinus officinalis) (REO) are known for their antimicrobial activity and are commonly used to extend the shelf life of many food products [20, 21]. Many studies have shown that the antimicrobial activity of a given essential oil can be ascribed to its main components and to the interaction between them [22]. Regarding meat products, OEO has been added to beef fillets [23], minced meat [24], chicken meat [25], fish meat [26], and ham [11]. Meanwhile, rosemary essential oil (REO) has been added to pork sausages [3], minced meat [27], and poultry fillets [28]. Despite the large amount of studies on the influence of essential oils on different meat products, there are not many studies that quantify the extension of the shelf life in terms of the concentration of EO added to each product.
The purpose of this study was to assess the impacts of different concentrations of essential oils of oregano and rosemary in the shelf life and some physicochemical parameters of vacuum-packed refrigerated Tuscan sausage.
Materials and methods
Extraction and characterization of the essential oils
Dehydrated oregano and rosemary leaves were purchased in the local commerce of the city of Chapecó, Brazil. The essential oils were extracted through steam distillation [29]. The samples were stored in amber glass flasks at 5.0 ± 1.0 °C.
To characterize the essential oils, gas chromatography/mass spectrometry analyses were conducted in a gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) coupled to a mass spectrometer (Agilent) using a capillary column HP-5MS (30 m × 0.25 mm × 0.25 μm composed of 5% phenylmethylpolysiloxane, J & W Scientific Inc., Folsom, CA, USA). The following temperature program was employed: 60 °C for 1 min, increased to 240 °C at 3 °C/min. Electron impact ionization at 70 eV with scan mass range of 45–400 m/z was used. Helium was used as carrier gas at 1 mL/min and the split mode at a ratio 1:20. Compounds were identified using digital libraries (NIST14). The percentage of each individual compound in relation to the total amount of detected substances was evaluated by the peak area gas chromatography (GC) normalization method. Analyses were conducted in triplicate.
Preparation, packaging, and storage of Tuscan sausage
The Tuscan sausage was prepared according to Brazilian legislation [30]. Forty-three kilograms of deboned pork and 13 kg of fat were obtained from retail sale, Santa Maria, RS, Brazil, grinded (8-mm diameter, Jamar PJ22 Grinder, Jamar Ltda., São Paulo, SP, Brazil) and mixed (Jamar MJI 35, Jamar) with other ingredients (Table 1). The mixture was homogenized for 5 min at 7 °C to a paste, cured for 30 min, and divided into nine batches (6.5 kg each). One batch was considered as control, and the other eight batches were added with OEO and REO at 0.05 wt%, 0.1 wt%, 0.2 wt%, and 0.4 wt%. Details of composition of each treatment are presented in Table 1.
Table 1.
Components (g/100 g) used in the formulation of the Tuscan sausage for each treatment adopted
| Ingredient | Control | OEO | REO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.05 wt% | 0.1 wt% | 0.2 wt% | 0.4 wt% | 0.05 wt% | 0.1 wt% | 0.2 wt% | 0.4 wt% | ||
| Pork meat (leg) | 71.50 | 71.45 | 71.40 | 71.30 | 71.10 | 71.45 | 71.40 | 71.30 | 71.10 |
| Pork fat | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 |
| Commercial mix Tuscan1 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Water | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Acidity regulator2 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| OEO | – | 0.05 | 0.10 | 0.20 | 0.40 | – | – | – | – |
| REO | – | – | – | – | – | 0.05 | 0.10 | 0.20 | 0.40 |
1Commercial mix Tuscan (Conditec Additives and Condiments©): sodium chloride (73.25%), sucrose (10%), natural spices (10%) (garlic, coriander, and red pepper), sodium tripolyphosphate (2.50%), monosodium glutamate (1.8%), sodium erythorbate (1.5%), sodium nitrite (0.8%), and sodium nitrate (0.15%)
2Acidity regulator (Engenutri ©): sodium lactate (57%), lactic acid (37%), citric acid (3%), ascorbic acid (1%), sodium ascorbate (1%), and natural spices (1%) (cardamom and anise)
The meat pastes were embedded in natural casings immersed in a 1% acetic acid solution for 15 min for hydration. The sausages were packed in vacuum-sealed polyethylene bags (Selovac 200B, São Paulo, SP, Brazil) and stored at 4.0 ± 0.5 °C for 56 days.
pH measurements and water activity
pH was measured at 0, 2, 4, 6, 8, 10, 12, 14, 20, 26, 32, 38, 44, 50, and 56 days of storage. The measurements were performed with a pH meter (MPA-210, TECNOPON, Piracicaba, SP, Brazil) using 10 g of sample mixed with 100 mL of distilled water. The water activity (aw) of the sausage was measured only at the beginning of storage with a water activity meter (Aqualab Series 4TEV, Decagon Devices Inc., Pullman, WA, USA). All the measurements were conducted in triplicate.
Color measurements
The International Commission on Illumination (CIE) color parameters reported as lightness (L*), redness (a*), and yellowness (b*) were recorded for all of the treatments and the control. Measurements were determined with a spectrophotometer (Konica Minolta CM-700D, Osaka, Japan) after 0, 7, 14, 21, 28, 35, 42, 49, and 56 days of storage. Readings were performed using diffuse illumination, with D65 as illuminant, 8° geometry, and 8-mm port size, specular component excluded. The parameters Chroma (C*) and Hue angle (h°) were calculated according to Eqs. (1) and (2), respectively:
| 1 |
| 2 |
LAB enumeration
LAB populations were estimated at 0, 2, 4, 6, 8, 10, 12, 14, 20, 26, 32, 38, 44, 50, and 56 days of storage, when 25-g aliquots was obtained from each treatment and batch, transferred to sterile plastic bags, and added to 225 mL of peptone water (0.1%, w/v, Merck, Darmstadt, Hessen, Germany). The mixtures were homogenized, tenfold diluted with peptone water (0.1%, w/v, Merck), and pour plated in duplicate in de Mann, Rogosa, and Sharpe (MRS, Liofilchem, Kasvi, Roseto, Italy) agar; the plates were overlaid with MRS agar (Liofilchelm, 30 mL per plate) and incubated at 37 °C for 48 h, when the formed colonies were enumerated and the results expressed in colony-forming unities per gram (CFU/g).
Kinetics of pH changes
An adapted version of the three-phase linear model (BUC) [31]was used to describe kinetics of pH changes for each treatment. Equation (3) shows the mathematical expression corresponding to the model:
| 3 |
where pH(t) corresponds to the pH of the sample t days after storage, pH0 stands for the initial pH, λ represents a lag before pH starts to drop during storage, ν is a rate of decrease in pH, pHmin corresponds to an asymptotic value of pH, and tmax represents the time at which pH reaches this asymptotic limit.
Shelf life modeling
The experimental growth data of the natural LAB microbiota were expressed as log CFU/g. A version of the Baranyi-Roberts growth model (BAR) [32] without lag was fitted to the experimental data to evaluate the following kinetic growth parameters: maximum specific growth rate, initial logarithmic count, and final logarithmic count. Two maximum LAB limits were considered to establish the shelf life: 6 and 7 log (CFU/g). According to the literature, LAB cause sensory changes, color changes, slime formation, gas formation, and unpleasant odors in the product above these limits, which make its consumption unfeasible [33]. Non-linear regression was performed through the nlsMicrobio and minpack.lm packages from the open-source software R v. 3.5.0 [34]. The parameterized version of the BAR growth model (without lag) used in this study is given in Eq. (4) and the net logarithmic increase after the treatment is given in Eq. (5).
| 4 |
| 5 |
where N(t) stands for the bacterial population (CFU/g) after t days of the start of the experiment, Nmax stands for the final bacterial population (CFU/g), N0 represents the initial bacterial population (CFU/g), μmax represents the maximum specific growth rate (days−1), and A is the net logarithmic growth increase (log(CFU/g)).
The estimated shelf life values were modeled through a modified version of the well-known Weibull function [35], as shown in Eq. (6).
| 6 |
where tSL (days) is the shelf life of the sample after the addition of the essential oil, tSL0 (days) corresponds to the shelf life of the Tuscan sausage without the addition of the essential oil, [EO] represents the concentration of essential oil, b (days) and c are empirical parameters. If the value of parameter c is higher than 1, the curve is concave. If c < 1, the curve is convex and if c = 1, the curve corresponds to a straight line.
The dependence of the maximum specific growth rate of the LAB on the essential oil concentration was described for a first-order kinetics model given in Eq. (7):
| 7 |
where μmax, 0 represents the maximum specific growth rate of the LAB for the samples without EO addition (days−1) and k is a rate constant. In Eq. (7), it can be seen that the fractional decrease of μmax after the addition of 0.1 wt% of EO is given by (1 − e−0.1k).
Statistical analysis
The fitting capability of the BAR growth model to describe the experimental data measured in the present study was evaluated through the following statistical indices: root mean square error (RMSE), mean absolute error (MAE), second-order Akaike information criterion (AICC), Bayesian information criterion (BIC), bias factor (BF), and accuracy factor (AF). Equations (8) to (13) provide the respective mathematical expressions.
| 8 |
| 9 |
| 10 |
| 11 |
| 12 |
| 13 |
In Eqs. (8) to (13), the symbol corresponds to the ith prediction, yi represents the ith observation, Lp stands for the likelihood function, p is the number of parameters of the model, and n is the number of experimental observations.
Results and discussion
Essential oils composition
Table 2 shows the composition of OEO and REO evaluated through GC analysis: 52 components were identified in the volatile fraction of OEO and 40 components in the volatile fraction of REO. The analysis of the chemical composition of the EOs is of paramount importance due to the large variation in the composition depending on a number of factors, such as geographical region, part of the plant, and environmental conditions [36, 37].
Table 2.
Chemical composition of the OEO and of the REO evaluated through GC analysis
| Composts |
1 (min) |
Weight percentage (wt%) | |
|---|---|---|---|
| OEO | REO | ||
| Hex-(2E)-enal | 3.75 | 0.26 | – |
| α-Pinene | 5.38 | 3.81 | 15.37 |
| Camphene | 5.75 | 0.44 | 5.36 |
| β-Pinene | 6.48 | 1.96 | 4.95 |
| p-Cymene | 7.90 | 15.72 | 1.66 |
| Limonene | 8.03 | – | 1.69 |
| 1,8-Cineole | 8.18 | 0.29 | 33.89 |
| γ-Terpinene | 9.04 | 46.33 | 0.61 |
| Terpinolene | 10.10 | 21.15 | 1.99 |
| Linalool | 10.52 | – | 1.09 |
| Camphor | 12.27 | – | 17.59 |
| Borneol | 13.10 | 2.32 | 6.94 |
| α-Terpineol | 14.14 | – | 4.03 |
| β-Caryophyllene | 23.57 | 7.74 | 4.83 |
1Experimental retention time (
The main components identified in OEO were γ-terpinene (46.3%), terpinolene (21.2%), p-cymene (15.7%), and β-caryophyllene (7.7%). Other authors have obtained equivalent results with a slightly different proportion of the main components [38, 39]. Meanwhile, the main components observed in REO were 1,8-cineole (33.9%), camphor (17.6%), α-pinene (15.4%), borneol (6.9%), and camphene (5.4%), corresponding to the composition observed in other studies conducted with the same plant [40, 41].
Initial characterization of the product
The following experimental results were obtained for the pH and water activity of the fresh sausage prior to all the treatments: pH = 6.14± 0.04 and aw = 0.966±0.001. The results are expressed as mean values±standard error. The low standard errors observed in the results show that the samples were very similar in composition. The pH of a fresh sausage is directly related to its color and flavor. Thus, it can be considered as another useful index to evaluate the sausage quality. In general, the pH of Tuscan sausage ranges between 5.90 and 6.20 [42, 43].
Kinetics of change of physical-chemical parameters
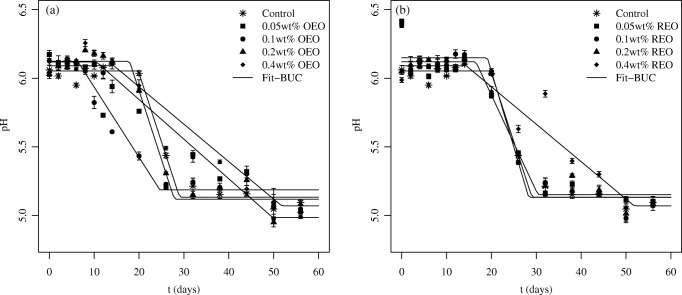
The pH of a given meat product is an important parameter that influences its characteristics because it affects many quality attributes, such as color, texture, water retention capacity, flavor, and microbial stability [44]. Figure 1 presents the curves obtained after the fit of Eq. (3) to the experimental data. The values of the fitted parameters can be seen in Table 3, that shows that the estimated results for the rate of decrease of pH (given by the parameter ν) were lower for the samples treated with a higher concentration (0.4 wt%) of EO, indicating that these treatments stabilized the pH of the sausages for a longer amount of time in relation to the other treatments. However, the asymptotic value of the pH (given by the parameter pHmin) obtained with this concentration of EO was not significantly lower in relation to the other treatments. This result shows that this treatment diminishes the rate of decrease of the pH, but it does not affect the final pH. In general, it is possible to visualize that the addition of the essential oils slows the decrease of the pH of the Tuscan sausage during storage for all treatments. However, it does not affect the asymptotic pH of the samples. The same behavior was observed after the addition of OEO to black wildebeest meat [45]. This result is associated with the stationary phase of microbial growth. Since the LAB are the main responsible for the pH change, if the number of viable cells remains constant, the pH does not change as well [33]. It was also observed that the lag before the drop in pH of the sausages was longer in the samples treated with REO when compared with the samples treated with OEO.
Fig. 1.
Kinetic profile of the pH of the treatments during storage
Table 3.
Fitted parameters (± 95% CI) and RMSE obtained after the fitting of the three-phase linear model (BUC) to the experimental data of pH obtained with each treatment
| Samples | (days) | pHmin | RMSE | ||
|---|---|---|---|---|---|
| Control | 6.05 (± 0.05) | 0.23 (± 0.07) | 19.84 (± 1.42) | 5.13 (± 0.06) | 0.051 |
| 0.05 wt% OEO | 6.12 (± 0.19) | 0.07 (± 0.02) | 4.94 (± 1.03) | 5.14 (± 0.19) | 0.125 |
| 0.1 wt% OEO | 6.12 (± 0.11) | 0.12 (± 0.06) | 6.36 (± 4.31) | 5.19 (± 0.09) | 0.089 |
| 0.2 wt% OEO | 6.12 (± 0.07) | 0.23 (± 0.11) | 17.87 (± 2.80) | 5.12 (± 0.09) | 0.076 |
| 0.4 wt% OEO | 6.12 (± 0.09) | 0.07 (± 0.02) | 10.50 (± 5.61) | 4.99 (± 0.15) | 0.081 |
| 0.05 wt% REO | 6.12 (± 0.08) | 0.16 (± 0.12) | 16.52 (± 5.67) | 5.15 (± 0.10) | 0.090 |
| 0.1 wt% REO | 6.15 (± 0.08) | 0.26 (± 0.12) | 19.10 (± 2.49) | 5.13 (± 0.10) | 0.088 |
| 0.2 wt% REO | 6.10 (± 0.06) | 0.16 (± 0.05) | 18.27 (± 2.96) | 5.14 (± 0.09) | 0.070 |
| 0.4 wt% REO | 6.09 (± 0.08) | 0.06 (± 0.02) | 14.37 (± 8.35) | 5.07 (± 0.23) | 0.090 |
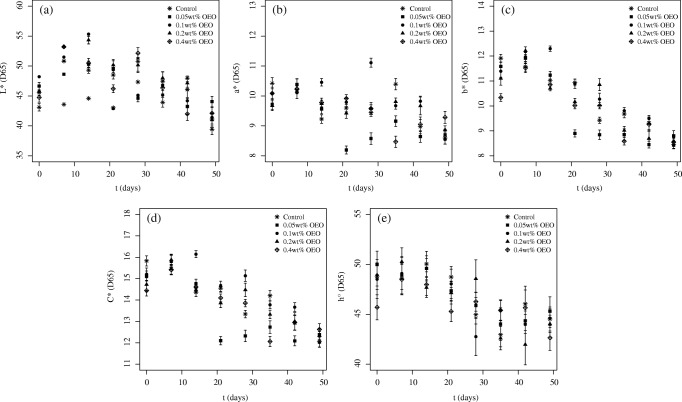
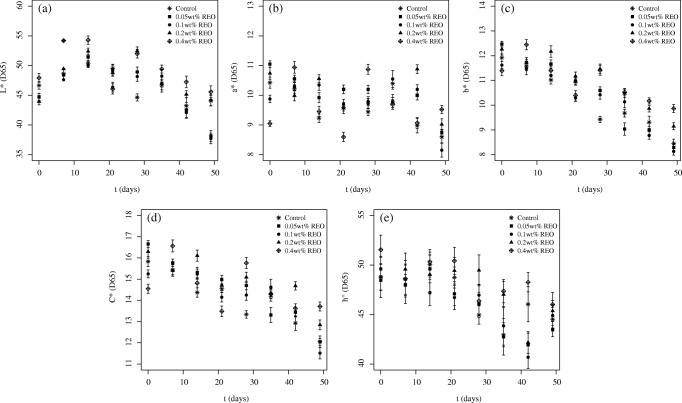
Results obtained for the kinetics of the color parameter changes are shown in Figs. 2 and 3 for the samples treated with OEO and REO, respectively. Both Figs. 2 and 3 only present values obtained until day 49 because results observed for color parameters after this period are no longer consistent due to spoilage of the sausage. It can be seen that the lightness increased in the first 14 days of storage for all of the samples, including the control, after which it decreased. A detailed analysis of values obtained for lightness must be considered cautiously, since some studies reported an increase in this parameter in minced beef treated with OEO after storage [46] and others described a decrease in the lightness over time [47]. The values of b* and C* decreased during storage, suggesting a browning of both the treated and untreated samples [46, 48]. By the end of storage, no difference was observed in the color parameters of the control and the samples with EO.
Fig. 2.
Kinetics of change in the color parameters for the different samples treated with OEO
Fig. 3.
Kinetics of change in the color parameters for the different samples treated with REO
Regarding the difference in the results obtained with samples treated with OEO and REO, the values of L* were generally higher for the samples treated with OEO in relation to REO. When lower doses of EO (0.05 wt% and 0.1 wt%) were added to the sausages, the values for the remaining parameters were equivalent in both EOs. However, after the addition of higher doses of EO (0.2 wt% and 0.4 wt%) the values were higher for OEO than for REO.
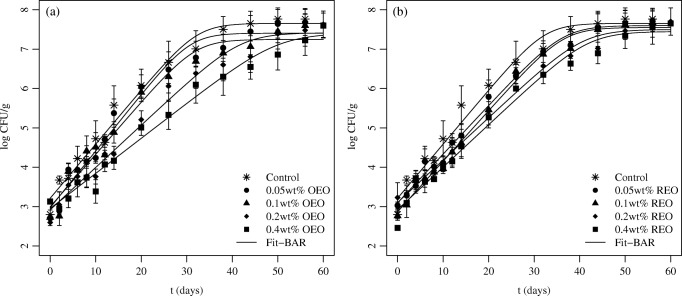
LAB consist in a substantial part of the natural microbiota of meat products. However, the formation of slimes and off-flavors as a result of their metabolic activity can make the consumption of the product inappropriate [13]. In the current study, two different LAB concentrations were adopted as microbiological limits for the sensory and microbiological quality of fresh sausages: 6 log (CFU/g) and 7 log (CFU/g). The period of time (in days) before the LAB concentration reached these limits was therefore adopted in the current study as the microbiological shelf life of the Tuscan sausage. After the fit of the BAR growth model to the LAB data, the microbiological shelf life of the Tuscan sausage was estimated with Eq. (4). Figure 4a presents the results obtained for the LAB concentration for OEO and Fig. 4b shows the results obtained for REO.
Fig. 4.
Experimental data and fitted growth curves of LAB in Tuscan sausage stored at 4.0 ± 0.5 °C added with different concentrations of oregano essential oil (OEO) (a) and rosemary essential oil (REO) (b)
The initial logarithmic count of the LAB for the different treatments ranged between 2.46 log (CFU/g) and 3.13 log (CFU/g). As can be seen in Fig. 4, no sharp lag phase was observed for any of the treatments used in this study. Since the LAB are part of the natural microbiota of the sausage, it is probable that the bacteria were previously adapted to the environment when the study was carried out. In addition, the net logarithmic bacterial increase given by the difference between the final and the initial bacterial load (parameter A) was not affected by the different treatments in relation to the control sample. Similarly, for the same reason, it was not observed a lag phase for LAB in vacuum-packed cooked meat emulsions at the storage temperatures of 0 °C, 8 °C, and 15 °C [49].
Figure 4 also shows that all treatments extended the shelf life of the Tuscan sausage in relation to the control. It can also be observed that the higher the concentration of EO added to the product, the longer the extension of the shelf life. A comparison of the results obtained for the different oils reveals that the shelf life estimated with REO was higher in relation to OEO when lower concentrations of EO (0.05 wt% and 0.1 wt%) were added to the sausage. On the other hand, when higher concentrations of oil (0.2 wt% and 0.4 wt%) were added to the sausage, the shelf life obtained with OEO was greater than with REO (Table 4).
Table 4.
Growth parameters (± 95% CI) estimated by fitting of the BAR growth model to the experimental data obtained with the different treatments in Tuscan sausage at 4.0 ± 0.5 °C
| Samples | log (log CFU/g) |
log Nmax (log CFU/g) |
A (log CFU/g) |
tSL (days) 7 log |
tSL (days) 6 log |
|
|---|---|---|---|---|---|---|
| Control | 0.316 (± 0.047) | 3.212 (± 0.267) | 7.654 (± 0.271) | 4.442 | 28.35 | 20.35 |
| 0.05 wt% OEO | 0.332 (± 0.061) | 2.971 (± 0.333) | 7.405 (± 0.317) | 4.434 | 29.47 | 21.14 |
| 0.1 wt% OEO | 0.307 (± 0.062) | 2.995 (± 0.345) | 7.246 (± 0.345) | 4.251 | 32.78 | 22.74 |
| 0.2 wt% OEO | 0.248 (± 0.032) | 2.919 (± 0.235) | 7.415 (± 0.297) | 4.496 | 39.90 | 28.80 |
| 0.4 wt% OEO | 0.205 (± 0.025) | 2.952 (± 0.215) | 7.405 (± 0.416) | 4.653 | 47.84 | 34.38 |
| 0.05 wt% REO | 0.285 (± 0.027) | 3.097 (± 0.174) | 7.599 (± 0.206) | 4.502 | 32.58 | 23.57 |
| 0.1 wt% REO | 0.296 (± 0.027) | 2.869 (± 0.176) | 7.550 (± 0.209) | 4.681 | 33.28 | 24.47 |
| 0.2 wt% REO | 0.248 (± 0.027) | 3.105 (± 0.236) | 7.550 (± 0.236) | 4.445 | 37.69 | 27.00 |
| 0.4 wt% REO | 0.248 (± 0.032) | 2.924 (± 0.240) | 7.447 (± 0.303) | 4.523 | 39.55 | 28.66 |
Table 4 summarizes the results obtained after the fit of the BAR growth model to the experimental data. As can be seen, the LAB population of the control sample reached the level of 6 log (CFU/g) after 20.35 days of storage and the level of 7 log (CFU/g) after 28.35 days of storage. Moreover, after this period of storage (20.35 and 28.35 days), the addition of 0.4 wt% of OEO and 0.4 wt% of REO resulted in a difference of approximately 1.5 log (CFU/g) in the LAB population in relation to the control (Fig. 4). This concentration of OEO extended the shelf life of the Tuscan sausage to 34.38 and 47.84 days, according to the first (7 log CFU/g of LAB) or second criterion (6 log CFU/g of LAB), respectively. In general, adding OEO will increase the shelf life of meat products. For instance, after the addition of 0.1 wt% of OEO in red porgy meat, a shelf life extension of 11 to 16 days was obtained in relation to the control treatment [27]. Regarding the REO, the shelf life extension was of 28.66 and 39.55 days for the first and second criteria, respectively. Even though the shelf life increased with the amount of oil added to the sausages, the application of lower concentrations (0.05 wt% and 0.1 wt%) of OEO or REO can be useful for practical applications. Actually, these doses do not significantly affect the sensory properties of different meat products [50, 51].
In general, a better fit was obtained when REO was added to the sausage instead of OEO. The addition of different concentrations of OEO and REO significantly affected the parameters μmax and tSL, as can be seen in Table 4. The maximum specific growth rate estimated for the non-treated sample was 0.316 days−1. A similar value for this parameter was obtained for untreated minced pork meat stored under modified atmosphere packaging at 5 °C [52]. The results obtained for the samples treated with 0.05 wt%, 0.1 wt%, 0.2 wt%, and 0.4 wt% of OEO were 0.332, 0.307, 0.248, and 0.205 days−1, respectively. On the other hand, the values of μmax obtained for the samples treated with 0.05 wt%, 0.1 wt%, 0.2 wt%, and 0.4 wt% of REO were 0.285, 0.296, 0.248, and 0.248 days−1, respectively. One could therefore conclude that although the maximum bacterial level observed after the treatment does not depend on the oil concentration (see results for log (Nmax) in Table 4), the LAB grew slower when lower concentrations of REO were added to the Tuscan sausage in relation to lower concentrations of OEO. The same analysis shows that their growth was slower when higher doses of OEO were added to the Tuscan sausage in relation to the same doses of REO.
According to the literature, the antimicrobial activity of OEO is related to the compounds γ-terpinene and p-cymene [53] and the antimicrobial activity of REO can be ascribed to the terpene derivatives and the 1,8-cineole naturally occurring in the essential oil [54, 55]. As a matter of fact, due to the lipophilic nature of the compounds present in the oils, they promote a rupture of the bacterial cell membrane and affect the cell’s vital functions. Both OEO and REO have been successfully applied to extend the shelf life of different meat products [56, 57]. The addition of OEO and REO to vacuum-packed fresh Bologna sausage stored during 24 days at 4 °C was also shown to reduce the LAB count [51, 52].
Table 5 exhibits the statistical indices (RMSE, MAE, AICC, BIC, BF, and AF) obtained after the fit of the BAR model to the experimental data. In general, RMSE and MAE are employed to measure model performance because they summarize the mean difference in the same units of the experimental data. The RMSE values obtained for all the studied treatments were lower than 0.274, suggesting a good fit of the BAR model to the experimental data. These results are of the same magnitude as those obtained with other studies that evaluated the natural microbiota of meat products, in which the RMSE ranged between 0.409 and 1.330 [58–60]. The bias and accuracy factors are commonly used statistical indexes in the field of predictive microbiology. A perfect agreement between observation and prediction results in bias and accuracy factors of 1. In the current study, the BF and AF values obtained for the different treatments ranged between 1.003–1.022 and 1.124–1.337, respectively, indicating that the model can describe the experimental data accurately.
Table 5.
Statistical indices obtained by the BAR growth model to the experimental data obtained with the different treatments in Tuscan sausage at 4.0 ± 0.5 °C
| Samples | Statistical indices | |||||
|---|---|---|---|---|---|---|
| RMSE | MAE | BIC | BF | AF | ||
| Control | 0.241 | 0.174 | 8.261 | 7.093 | 1.014 | 1.218 |
| 0.05 wt% OEO | 0.260 | 0.203 | 14.195 | 13.028 | 1.019 | 1.281 |
| 0.1 wt% OEO | 0.274 | 0.228 | 15.729 | 14.562 | 1.022 | 1.337 |
| 0.2 wt% OEO | 0.211 | 0.154 | 7.569 | 6.660 | 1.014 | 1.223 |
| 0.4 wt% OEO | 0.202 | 0.147 | 4.452 | 5.377 | 1.009 | 1.214 |
| 0.05 wt% REO | 0.145 | 0.119 | − 3.334 | − 4.502 | 1.005 | 1.124 |
| 0.1 wt% REO | 0.148 | 0.126 | − 2.844 | − 4.012 | 1.005 | 1.131 |
| 0.2 wt% REO | 0.172 | 0.150 | 1.148 | 0.239 | 1.003 | 1.153 |
| 0.4 wt% REO | 0.214 | 0.192 | 8.136 | 7.226 | 1.018 | 1.218 |
After the fit of Eq. (6) to the estimated values of the shelf life for all treatments, the following empirical expressions were obtained to describe the microbiological shelf life as a function of the oil concentration when the natural LAB microbiota assumed as microbiological limit is equal to 7 log (CFU/g). These equations can provide useful insights concerning the amount of oil that can be added to the sausage in order to increase its shelf life.
| 14 |
| 15 |
where tSLO and tSLR represent the microbiological shelf life of the Tuscan sausage treated with oregano and rosemary, respectively, [OEO] and [REO] correspond to the concentrations (wt%) of OEO and REO, respectively.
Equations (16) and (17) provide the empirical expressions corresponding to a microbiological limit of 6 log (CFU/g).
| 16 |
| 17 |
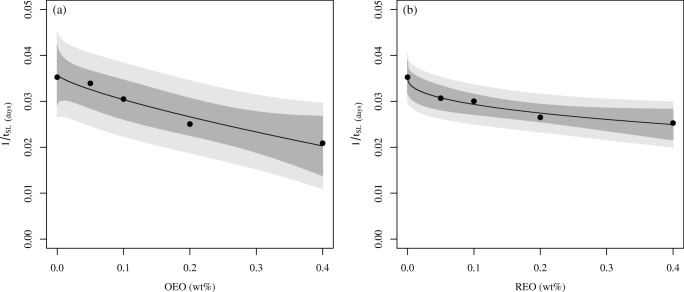
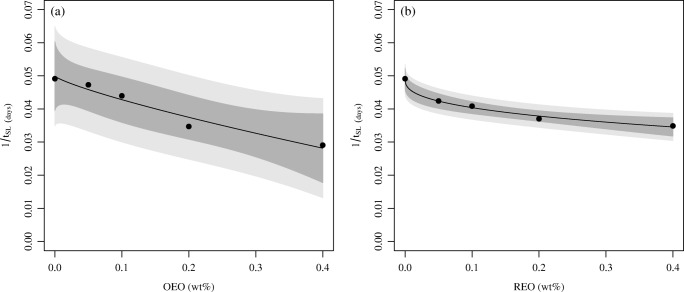
Figures 5 and 6 present the curves obtained after the fit of the modified Weibull model to the data of the shelf life. Because parameter c is less than one for all the curves (see Eqs. (14) to (17)), it can be concluded that all of them are convex in shape. Figures 5 and 6 show that when a lower concentration of EO is added to the sausages, a small amount of REO leads to a larger increase in the shelf life in relation to the OEO. However, when higher doses of EO are added to the Tuscan sausage, the increase of the shelf life becomes less pronounced, as can be seen in the asymptotic behavior of the curves. The predictions obtained were accurate, as can be easily observed in Table 6.
Fig. 5.
Microbiological shelf life of Tuscan sausage as a function of EO concentration obtained for the treatments considering as the microbiological limit a concentration of LAB equal to 7 log CFU/g. Dark shadow represents 95% CI and light shadow represents 95% prediction interval
Fig. 6.
Microbiological shelf life of Tuscan sausage as a function of EO concentration obtained for the treatments considering as the microbiological limit a concentration of LAB equal to 6 log CFU/g. Dark shadow represents 95% CI and light shadow represents 95% prediction interval
Table 6.
Statistical indices obtained after fit of Eq. 3 to the estimated values of the shelf life according to the different criteria adopted as microbiological limits
| Samples | Statistical indices | ||||||
|---|---|---|---|---|---|---|---|
| AIC | BIC | RMSE | MAE | AF | BF | ||
| 7 log (CFU/g) | OEO | 21.500 | 19.938 | 0.933 | 0.847 | 1.140 | 1.003 |
| REO | 18.135 | 16.572 | 0.667 | 0.511 | 1.079 | 1.001 | |
| 6 log (CFU/g) | OEO | 20.602 | 19.040 | 0.853 | 0.774 | 1.180 | 1.004 |
| REO | 9.482 | 7.922 | 0.281 | 0.209 | 1.043 | 1.003 | |
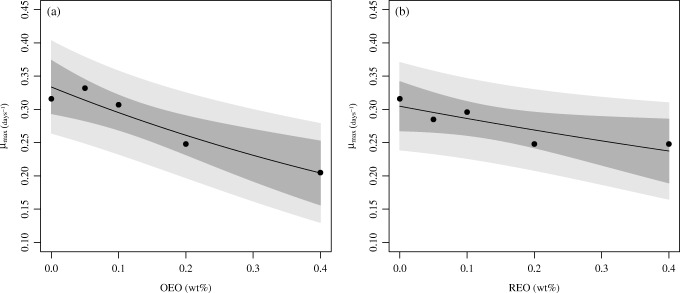
Figure 7 shows the fit of the first-order kinetics model, given in Eq. (7), to the data of the maximum specific growth rate as a function of the concentration of essential oil. Table 7 shows the results obtained for the fitted parameters. Table 7 reveals that as the amount of EO added to the sausages increases, there is a larger decrease in the growth of the native LAB in the sausages. According to the values of the parameter k, on average, the addition of 0.1 wt% of OEO slows LAB growth at about 11.5% and the addition of REO slows LAB growth at about 6.0%. This result does not consider if smaller or higher doses of EO were added; it takes into account the overall behavior of the data.
Fig. 7.
Maximum specific growth rate as a function of EO concentration. Dark shadow represents 95% CI and light shadow represents 95% prediction interval
Table 7.
Fitted parameters (± 95% CI) and RMSE obtained after the fitting of the first-order kinetics model to the estimated data of maximum specific growth rate
| Samples | k | RMSE | MAE | |
|---|---|---|---|---|
| OEO | 0.334 (± 0.013) | 1.225 (± 0.235) | 0.014 | 0.012 |
| REO | 0.305 (± 0.116) | 0.624 (± 0.210) | 0.013 | 0.012 |
Conclusions
This work explored the potential of the addition of the essential oils from oregano and rosemary to extend the microbiological shelf life of vacuum-packed Tuscan sausage. Both rosemary and oregano oils are alternatives to conventional preservatives and resulted in a larger increase in the shelf life in relation to the control. The advantages derived from the use of oregano and rosemary essential oils make them promising alternatives for the preservation of Tuscan sausage due to their effectiveness in slowing the growth of lactic acid bacteria, which are the main culprits for the spoilage of many meat products, in addition to reducing the reliance on chemical and synthetic additives commonly used in these products.
Funding information
The authors express their gratitude to FAPESC (Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina, grant no. 2017TR727) for financial support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brazil (2016) Ministry of Agriculture, Livestock and Food Supply (MAPA), http://www.brasil.gov.br/economia-e-emprego/2016/03/brasil-e-eua-renegociam-mercado-de-carnebovina. Accessed 05 May 2019
- 2.Brazil (2017) Annual Report 2018. Brazilian Association of Animal Protein (ABPA), http://abpa-br.com.br/setores/suinocultura/publicacoes/relatorios-anuais/2018. Accessed 05 May 2019
- 3.Georgantelis D, Ambrosiadis I, Katikou P, Blekas G, Georgaki SA. Effect of rosemary extract, chitosan and α-tocopherol on microbiological parameters and lipid oxidation of fresh pork sausages stored at 4 °C. Meat Sci. 2007;76(1):172–181. doi: 10.1016/j.meatsci.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Šojić B, Pavli B, Zekovi Z, Tomovi V, Ikoni P, Natalija D. The effect of essential oil and extract from sage (Salvia officinalis L.) herbal dust (food industry by-product) on the oxidative and microbiological stability of fresh pork sausages. LWT - Food Sci Technol. 2018;89(9):749–755. [Google Scholar]
- 5.Borch E, Kant-Muermans ML, Blixt Y. Bacterial spoilage of meat and cured meat products. Int J Food Microbiol. 1996;33(1):103–120. doi: 10.1016/0168-1605(96)01135-x. [DOI] [PubMed] [Google Scholar]
- 6.Hu P, Zhou G, Xu X, Li C, Han Y. Characterization of the predominant spoilage bacteria in sliced vacuum-packed cooked ham based on 16S rDNA-DGGE. Food Control. 2009;20(2):99–104. [Google Scholar]
- 7.Paula R, Colet R, Oliveira D, Valduga E, Treichel H. Assessment of different packaging structures in the stability of frozen fresh Brazilian Toscana sausage. Food Bioprocess Technol. 2011;4(3):481–485. [Google Scholar]
- 8.Audenaert K, Haene KD, Messens K, Ruyssen T, Vandamme P, Huys G. Diversity of lactic acid bacteria from modified atmosphere packaged sliced cooked meat products at sell-by date assessed by PCR-denaturing gradient gel electrophoresis. Food Microbiol. 2010;27(1):12–18. doi: 10.1016/j.fm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Hugo CJ, Hugo A. Current trends in natural preservatives for fresh sausage products. Trends Food Sci Technol. 2015;45(1):12–23. [Google Scholar]
- 10.Zhou GH, Xu XL, Liu Y. Preservation technologies for fresh meat – a review. Meat Sci. 2010;86(1):119–128. doi: 10.1016/j.meatsci.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 11.Menezes NMC, Martins WF, Longhi DA, Aragão GMF. Modeling the effect of oregano essential oil on shelf-life extension of vacuum-packed cooked sliced ham. Meat Sci. 2018;139(9):113–119. doi: 10.1016/j.meatsci.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes PAR, Moreira SA, Duarte R, Santos DI, Queirós RP, Fidalgo LG, Santos MD, Delgadillo I, Saraiva JA. Preservation of sliced cooked ham at 25, 30, and 37 °C under moderated pressure (hyperbaric storage) and comparison with refrigerated storage. Food Bioprod Process. 2015;95:200–207. [Google Scholar]
- 13.Labadie J. Consequences of packaging on bacterial growth. Meat is an ecological niche. Meat Sci. 1999;52(3):299–305. doi: 10.1016/s0309-1740(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 14.International Commission on Microbiological Specifications for Foods (ICMSF) Microorganisms in foods microbial ecology of food commodities. London: Blackie Academic & Professional; 1998. [Google Scholar]
- 15.Bouju-Albert A, Pilet MF, Guillou S. Influence of lactate and acetate removal on the microbiota of French fresh pork sausages. Food Microbiol. 2018;76:328–336. doi: 10.1016/j.fm.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Sultana T, Rana J, Chakraborty SR, Das KK, Rahman T, Noor R. Microbiological analysis of common preservatives used in food items and demonstration of their in vitro antibacterial activity. Asian Pacific J Trop Dis. 2014;4(6):453–456. [Google Scholar]
- 17.Jayasena DD, Jo C. Essential oils as potential antimicrobial agents in meat and meat products: a review. Trends Food Sci Technol. 2013;34(2):96–108. [Google Scholar]
- 18.Calo JR, Crandall PGO, O’Bryan CA, Ricke SC. Essential oils as antimicrobials in food systems – a review. Food Control. 2015;54(8):111–119. [Google Scholar]
- 19.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils - a review. Food Chem Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 20.McMillin KW. Advancements in meat packaging. Meat Sci. 2017;132:153–162. doi: 10.1016/j.meatsci.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro-Santos R, Andrade M, Sanches-Silva A, Melo NR. Essential oils for food application: natural substances with established biological activities. Food Bioprocess Technol. 2018;11(1):43–71. [Google Scholar]
- 22.Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012;3(1):1–24. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsigarida E, Skandamis P, Nychas GJE. Behaviour of listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5°C. J Appl Microbiol. 2000;89(6):901–909. doi: 10.1046/j.1365-2672.2000.01170.x. [DOI] [PubMed] [Google Scholar]
- 24.Govaris A, Solomakos N, Pexara A, Chatzopoulou PS. The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. Int J Food Microbiol. 2010;137(2–3):175–180. doi: 10.1016/j.ijfoodmicro.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Ntzimani AG, Giatrakou VI, Savvaidis IN. Combined natural antimicrobial treatments (EDTA, lysozyme, rosemary and oregano oil) on semi cooked coated chicken meat stored in vacuum packages at 4 °C: microbiological and sensory evaluation. Innov Food Sci Emerg Technol. 2010;11(1):187–196. [Google Scholar]
- 26.Vatavali K, Karakosta L, Nathanailides C, Georgantelis D, Kontominas MG. Combined effect of chitosan and oregano essential oil dip on the microbiological, chemical, and sensory attributes of red porgy (Pagrus pagrus) stored in ice. Food Bioprocess Technol. 2013;6(12):3510–3521. [Google Scholar]
- 27.Amariei S, Poroch-Seriţan M, Gutt G, Oroian M, Ciornei E. Rosemary, thyme and oregano essential oils influence on physicochemical properties and microbiological stability of minced meat. J Microbiol Biotechnol Food Sci. 2016;6(1):670–676. [Google Scholar]
- 28.Kahraman T, Issa G, Bingol EB, Kahraman BB, Dumen E. Effect of rosemary essential oil and modified-atmosphere packaging (MAP) on meat quality and survival of pathogens in poultry fillets. Braz J Microbiol. 2015;46(2):591–599. doi: 10.1590/S1517-838246220131201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chemat F, Boutekedjiret C (2015) Extraction // steam distillation. In Reference Module in Chemistry. Mol Sci Chem Eng
- 30.Brazil Ministry of Agriculture, Livestock and Food Supply (MAPA), Secretariat for the Defense of Agribusiness (SDA). Normative Instruction no. 04, March 31, 2000. Technical regulation of identity and quality of mechanically separated meat and sausages. Off J Fed Republic Brazil. 2000;1:6–10. [Google Scholar]
- 31.Buchanan RL, Whiting RC, Damert WC. When is simple good enough: a comparison of the Gompertz, Baranyi, and three-phase linear models for fitting bacterial growth curves. Food Microbiol. 1997;14(4):313–326. [Google Scholar]
- 32.Baranyi J, Roberts TA. A dynamic approach to predict bacterial growth in food. Int J Food Microbiol. 1994;23(1):277–294. doi: 10.1016/0168-1605(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 33.Pothakos V, Devlieghere F, Villani F, Björkroth J, Ercolini D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015;109(2015):66–74. doi: 10.1016/j.meatsci.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 34.R Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 35.Mafart P, Couvert O, Gaillard S, Leguerinel I. On calculating sterility in thermal preservation methods: application of the Weibull frequency distribution model. Int J Food Microbiol. 2002;72(1–2):107–113. doi: 10.1016/s0168-1605(01)00624-9. [DOI] [PubMed] [Google Scholar]
- 36.Reza M, Alireza S, Nazeri V, Maggi F, Craker L. Essential oil profile of oregano (Origanum vulgare L.) populations grown under similar soil and climate conditions. Ind Crop Prod. 2018;119(3):183–190. [Google Scholar]
- 37.Sirocchi V, Devlieghere F, Peelman N, Sagratini G, Maggi F, Vittori S, Ragaert P. Effect of Rosmarinus officinalis L. essential oil combined with different packaging conditions to extend the shelf life of refrigerated beef meat. Food Chem. 2017;221:1069–1076. doi: 10.1016/j.foodchem.2016.11.054. [DOI] [PubMed] [Google Scholar]
- 38.Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85(4):633–640. [Google Scholar]
- 39.Bendahou M, Muselli A, Grignon-Dubois M, Benyoucef M, Desjobert JM, Bernardini AF, Costa J. Antimicrobial activity and chemical composition of Origanum glandulosum Desf. Essential oil and extract obtained by microwave extraction: comparison with hydrodistillation. Food Chem. 2008;106(1):132–139. [Google Scholar]
- 40.Jiang Y, Wu N, Fu Y, Wang W, Luo M. Chemical composition and antimicrobial activity of the essential oil of rosemary. Environ Toxicol Pharmacol. 2011;32(1):63–68. doi: 10.1016/j.etap.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Sirocchi V, Caprioli G, Cecchini C, Coman MM, Cresci A, Maggi F, Sagratini G. Biogenic amines as freshness index of meat wrapped in a new active packaging system formulated with essential oils of Rosmarinus officinalis. Int J Food Sci Nutr. 2013;64(8):921–928. doi: 10.3109/09637486.2013.809706. [DOI] [PubMed] [Google Scholar]
- 42.Silveira SM, Luciano FB, Fronza N, Cunha A, Jr, Scheuermann GN, Vieira CRW. Chemical composition and antibacterial activity of Laurus nobilis essential oil towards foodborne pathogens and its application in fresh Tuscan sausage stored at 7 °C. LWT - Food Sci Technol. 2014;59(1):86–93. [Google Scholar]
- 43.Glorieux S, Steen L, Van de Walle D, Dewettinck K, Foubert I, Fraeye I. Effect of meat type, animal fat type, and cooking temperature on microstructural and macroscopic properties of cooked sausages. Food Bioprocess Technol. 2019;12(1):16–26. [Google Scholar]
- 44.Lee MA, Choi JH, Choi YS, Han DJ, Kim HY, Shim SY, Kim CJ. The antioxidative properties of mustard leaf (Brassica juncea) kimchi extracts on refrigerated raw ground pork meat against lipid oxidation. Meat Sci. 2010;84(3):498–504. doi: 10.1016/j.meatsci.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Shange M, Makasi T, Gouws P, Hoffman LC. Preservation of previously frozen black wildebeest meat (Connochaetes gnou) using oregano (Oreganum vulgare) essential oil. Meat Sci. 2019;148:88–95. doi: 10.1016/j.meatsci.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Hulankova R, Borilova G, Steinhauserova I. Combined antimicrobial effect of oregano essential oil and caprylic acid in minced beef. Meat Sci. 2013;95(2):190–194. doi: 10.1016/j.meatsci.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Chouliara E, Karatapanis A, Savvaidis IN, Kontominas MGA. Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4 °C. Food Microbiol. 2007;24(6):607–617. doi: 10.1016/j.fm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Bonilla J, Vargas M, Atarés L, Chiralt A. Effect of chitosan essential oil films on the storage-keeping quality of pork meat products. Food Bioprocess Technol. 2014;7(8):2443–2450. [Google Scholar]
- 49.Cayre ME, Garro O, Vignolo G. Effect of storage temperature and gas permeability of packaging film on the growth of lactic acid bacteria and Brochothrix thermosphacta in cooked meat emulsions. Food Microbiol. 2005;22(6):505–512. [Google Scholar]
- 50.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez JA. Effect of orange dietary fibre, oregano essential oil and packaging conditions on shelf-life of bologna sausages. Food Control. 2010;21(4):436–443. [Google Scholar]
- 51.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez JA. Effect of adding citrus fibre washing water and rosemary essential oil on the quality characteristics of a bologna sausage. LWT - Food Sci Technol. 2010;43(6):958–963. [Google Scholar]
- 52.Dissing BS, Papadopoulou OS, Tassou C, Ersboll BK, Carstensen JM, Panagou EZ, Nychas GJ. Using multispectral imaging for spoilage detection of pork meat. Food Bioprocess Technol. 2013;6(9):2268–2279. [Google Scholar]
- 53.Burt S. Essential oils: their antibacterial properties and potential applications in foods - a review. Int J Food Microbiol. 2004;94(14):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 54.Mourey A, Canillac N. Anti-listeria monocytogenes activity of essential oils components of conifers. Food Control. 2002;13(4–5):289–292. [Google Scholar]
- 55.Realini CE, Marcos B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014;98(3):404–419. doi: 10.1016/j.meatsci.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 56.Rosato A, Vitali C, De Laurentis N, Armenise D, Milillo M. Antibacterial activity of some essential oils administered alone or in combination with norfloxacin. Phytomed. 2007;14(11):727–732. doi: 10.1016/j.phymed.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Barbosa LN, Probst IS, Murbach Teles Andrade BF, Bérgamo Alves FC, Albano M, Mores Rall VL, Júnior AF. Essential oils from herbs against foodborne pathogens in chicken sausage. J Oleo Sci. 2015;64(1):117–124. doi: 10.5650/jos.ess14163. [DOI] [PubMed] [Google Scholar]
- 58.Paparella A, Mazzarrino G, Chaves-López C, Rossi C, Sacchetti G, Guerrieri O, Serio A. Chitosan boosts the antimicrobial activity of Origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol. 2016;59:23–31. doi: 10.1016/j.fm.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 59.Kreyenschmidt J, Hübner A, Beierle E, Chonsch L, Scherer A, Petersen B. Determination of the shelf life of sliced cooked ham based on the growth of lactic acid bacteria in different steps of the chain. J Appl Microbiol. 2010;108(2):510–520. doi: 10.1111/j.1365-2672.2009.04451.x. [DOI] [PubMed] [Google Scholar]
- 60.Silva NB, Longhi DA, Martins WF, Laurindo JB, Aragão GMF, Carciofi BA. Modeling the growth of Lactobacillus viridescens under non-isothermal conditions in vacuum-packed sliced ham. Int J Food Microbiol. 2017;240(1):57–62. doi: 10.1016/j.ijfoodmicro.2016.05.014. [DOI] [PubMed] [Google Scholar]