Figure 1: Mechanism underlying the anti-glioma immune response following TK/Flt3L gene therapy.
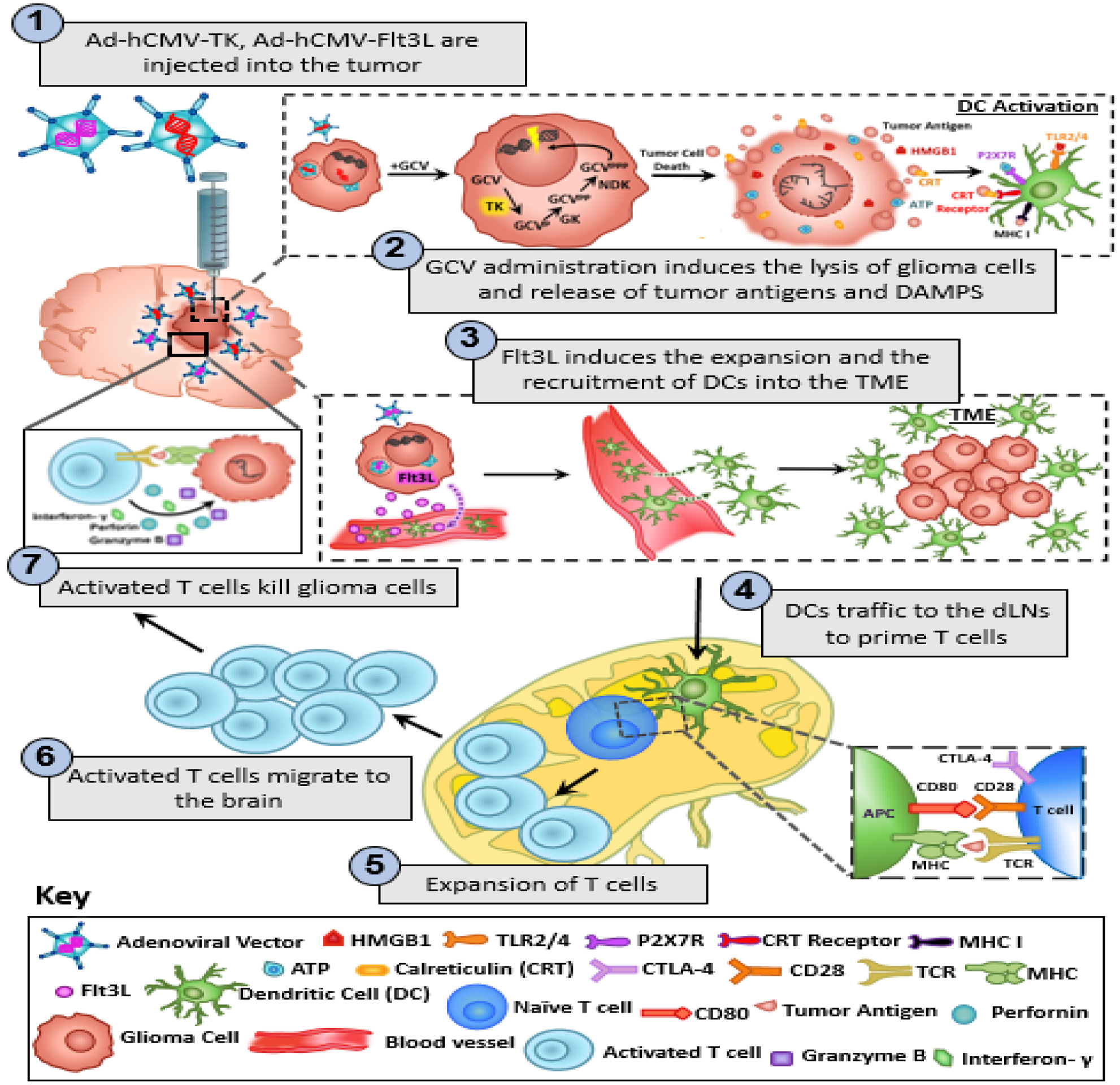
First generation adenoviral vectors encoding HSV1-Thymidine Kinase (TK) and HSV1-Flt3L are intratumorally injected. This is followed by systemic administration of prodrug ganciclovir (GCV). TK is capable of converting GCV to GCV-triphosphate, a purine analog that selectively inhibits DNA replication in proliferating tumor cells. The expression of TK in the presence of GCV mediates the release of damage associated molecular patterns (DAMPs), i.e. HMBG1, Calreticulin, and ATP from dying tumor cells. Expression of Flt3L recruits dendritic cells (DCs) into the tumor milieu where they take up brain tumor antigens released from the dying glioma cells and present them on their MHC complexes. HMGB1 binds to TLR2/4, which promotes the production of cytokines and tumor antigen cross-presentation. The binding of extracellular ATP to purigenic receptor P2X7R promotes the recruitment of DCs to the tumor milieu. The DCs loaded with tumor antigens migrate to the cervical draining lymph nodes where they present tumor antigens to naïve T cells, priming tumor specific anti-glioma effector T cells. The tumor specific effector T cells then migrate back into the brain and kill residual glioma cells via the production of granzyme B, perforin and effector cytokine IFN-y.
