Figure 1: UVB- and UVA-induced melanogenic signaling in epidermal melanocytes.
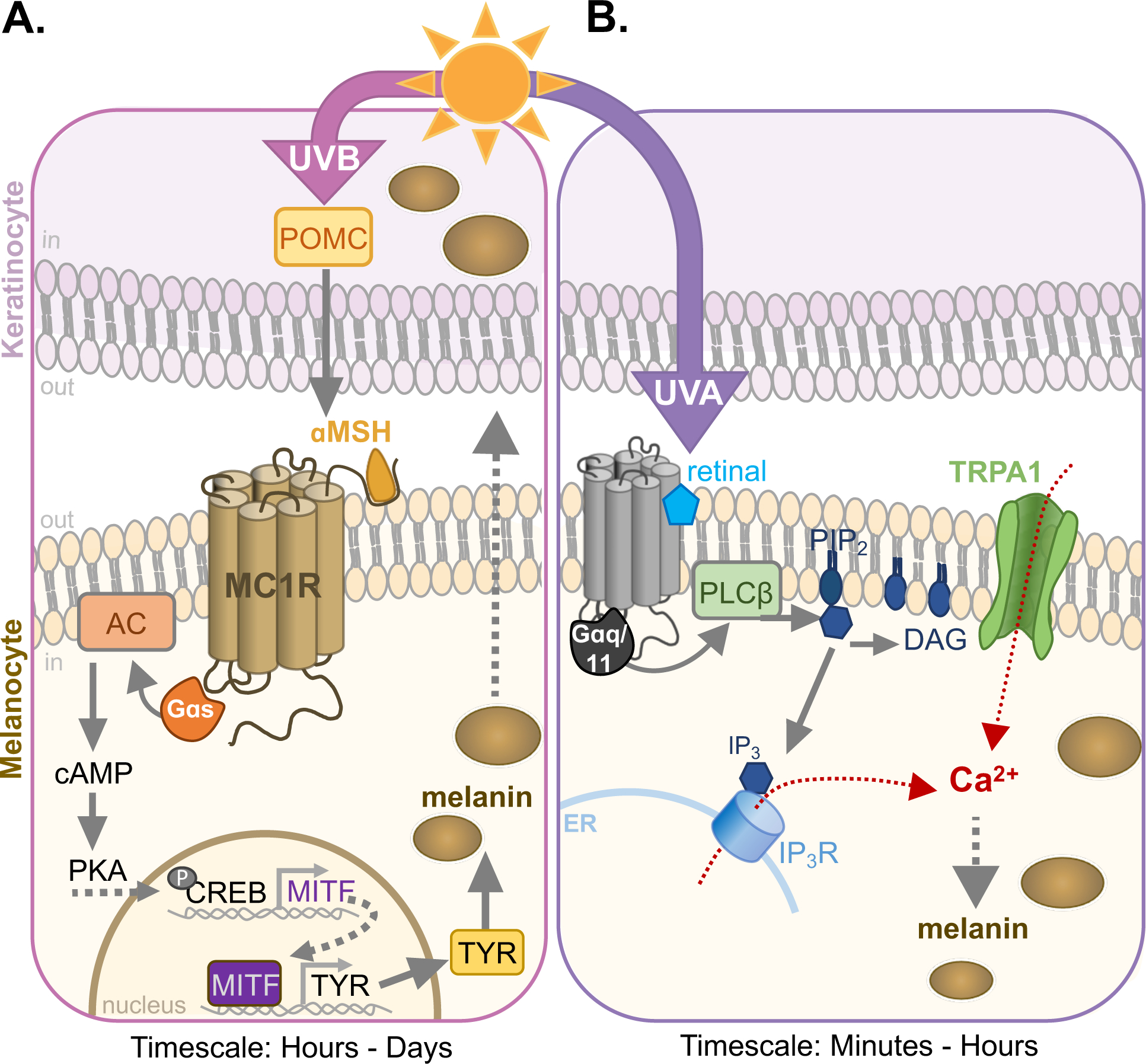
A. UVB induces DNA damage, causing the release of pro-opiomelanocortin (POMC) by keratinocytes and melanocytes. POMC is cleaved to αMSH, the endogenous agonist for the melanocortin-1 receptor (MC1R) in melanocytes. Activation of MC1R signals via Gαs to increase intracellular cAMP levels through activation of adenylyl cyclase (AC). cAMP renders protein kinase A (PKA) catalytically active, phosphorylating cAMP response element binding protein (CREB) which upregulates microphthalmia-associated transcription factor (MITF). The subsequent MITF-driven increase in expression of melanogenic enzymes like tyrosinase (TYR) leads to an increase in melanin within melanosomes (brown circles) and melanin transfer to keratinocytes, which occurs in 12–24 h after UVB exposure.
B. UVA, detected by an as yet unidentified melanocyte photoreceptor, leads to retinal-dependent activation of Gαq/11, which stimulate phospholipase C-β (PLCβ) to hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol triphosphate (IP3). IP3 binds its cognate receptor IP3R, increasing Ca2+ flux from the endoplasmic reticulum (ER). This pathway also activates transient receptor potential cation channel A1 (TRPA1), leading to Ca2+ influx and a further increase in intracellular Ca2+. Ca2+ from both sources is required for the increase melanin synthesis that can be detected 1 h after exposure (Bellono et al., 2014; 2013; Wicks et al., 2011).
