Figure 5.
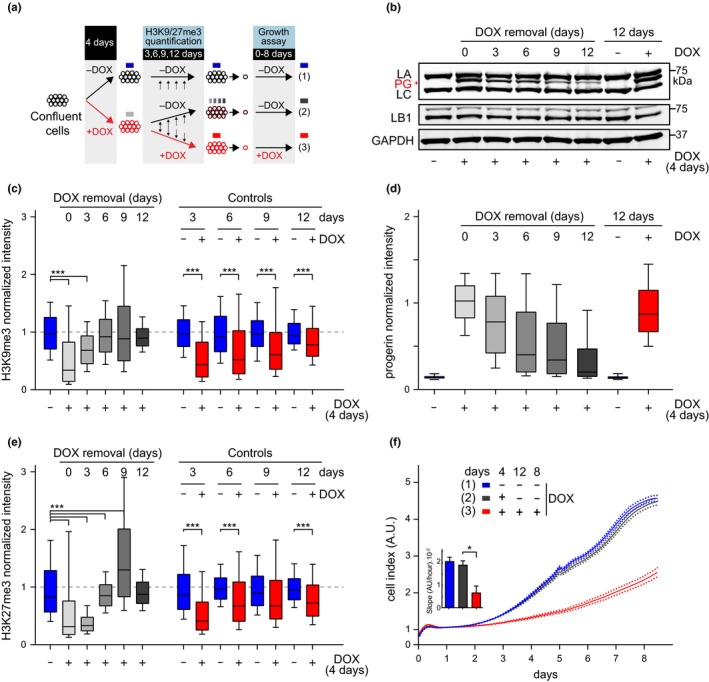
Restored heterochromatin levels and cell proliferation upon progerin removal in G1‐arrested NDF. (a) NDF were grown to confluence and exposed to progerin expression (+DOX, red circles) for 4 days. Nonexposed cells (−DOX, black circles, blue box) served as a control. After 4 days, progerin expression was either turned off (−DOX; black/red circles, grey box) or left on (+DOX, red circles, red box) and kept confluent for an additional 12 days. Proliferation rates were compared for cells never exposed to progerin (blue), cells in which progerin was removed (grey) and cells constitutively exposed to progerin (red). (b) Western blotting showing doxycycline‐dependent progerin expression and removal in confluent primary fibroblasts. Progerin (PG), lamin A (LA) and lamin C (LC), lamin B1 (LB1) and GAPDH are indicated. Progerin intensities quantified by single‐cell immunofluorescence microscopy at each day are indicated in panel (d). A total of ~ 7.7 × 104 nuclei were quantified for both H3K9me3 and H3K27me3 quantifications, from 2 independent experiments. Whiskers represent 10–90 percentile. (c,e) Box plot representation of H3K9me3 (c) and H3K27me3 (e) levels at the indicated times post doxycycline removal (0, 3, 6, 9, 12 days; grey bars) or in controls never (blue) or constitutively (red) exposed to progerin. A total of ~3.8 × 104 and ~3.9 × 104 nuclei were quantified for H3K9me3 and H3K27me3, respectively, from 2 independent experiments, ***p < .001, one‐way ANOVA with Sidak's post‐test. Whiskers represent 10–90 percentile. (d) Box plot representation of progerin normalized intensity per nucleus, in NDF induced to express progerin (grey, red) or noninduced (blue/black). (f) Growth curve of control and primary fibroblasts continuously expressing progerin (red line), not expressing progerin (blue line), or expressing progerin for 4 days while confluent and without induction thereafter (black). Dotted lines indicate SEM (n = 3). Inset: growth rate after 8 days, error bars indicate SEM (*p < .05, one‐way ANOVA with Tukey's post‐test)
