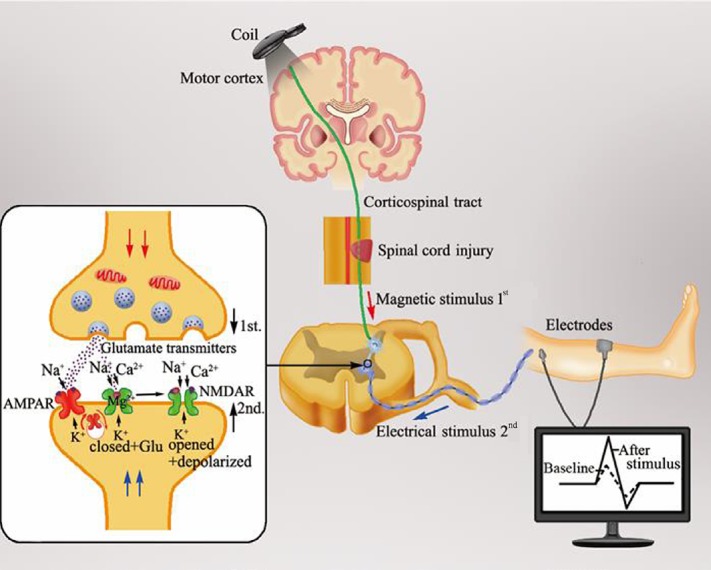
Figure 1.
Change of synaptic plasticity in the spinal cord following a neuromodulation stimulation mode called spike-timing dependent plasticity.
When electrical impulses by transcranial magnetic stimulation over the M1 region passes through the corticospinal tract to the presynaptic membrane of the downstream neuron in the spinal cord anterior horn controlling the targeted muscle, the glutamate released from the presynaptic membrane binds to the postsynaptic membrane of the motor neuron. After a delay of 1–2 ms, the electrical signal transmitted to the postsynaptic membrane generated from the electrical stimulus on the peripheral nerve forms an inverse synchronous stimulus at the anterior horn of the spinal cord. This inverse stimulus depolarizes the postsynaptic membrane and the Mg2+ block is removed with the Ca2+ influx through the N-methyl-D-aspartic acid receptor. As a result, the change of intracellular Ca2+ concentration resulting from the Ca2+ influx reduces the excitability threshold of the postsynaptic membrane and enhances the induction possibility of long-term potentiation effects, with an increased motor evoked potential amplitude, as revealed by electromyography. Hence, spike-timing dependent plasticity with a synchronous stimulus in the anterior horn efficiently enhances synaptic plasticity in the spinal cord.