Figure 1.
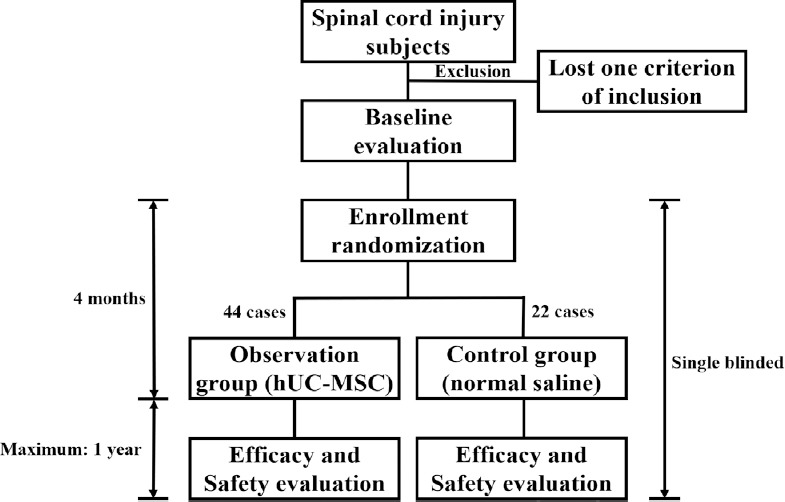
Schematic diagram of the use of human umbilical cord mesenchymal stem cells (hUC-MSC) to treat early chronic spinal cord injury.
Following baseline assessments, enrolled spinal cord injury patients at between 2 months and 1 year post-injury will be randomly allocated into one of two groups: the observation group (subarachnoid transplantation of hUC-MSC, n = 44) and the control group (intrathecal infusion of sterile normal saline, n = 22). In this single-blinded study, both groups will receive four doses of subarachnoid administration, with an interval of one calendar month between each administration. After completing the cytotherapy, regular follow-ups for both safety and efficacy will be performed at four time points, scheduled at 1, 3, 6, and 12 months post-cytotherapy.
