Figure 5.
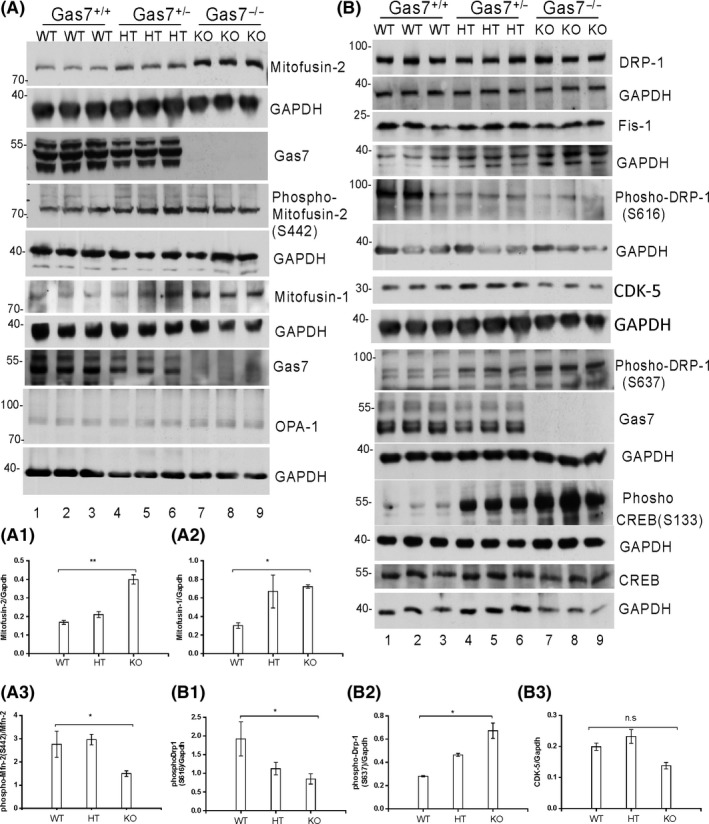
Profusion and profission inhibitor protein levels are increased in gas7‐knockout mouse cerebrum. A, Western blot analysis. Total brain lysates from wild‐type (WT), gas7 heterozygous (HT), and gas7‐knockout (KO) mice were electrophoresed by 10% SDS‐PAGE. After blotting, the membranes were probed with Gas7 antibody and show that Gas7 protein exists in wild‐type and gas7 heterozygotes but not in gas7‐knockout mice. Blots detecting Mitofusin‐2, phospho‐mitofusin‐2 (S442), Mitofusin‐1, and Opa‐1 from cerebrum lysates of Gas7 WT, HT, and KO mice show increased mitofusin proteins in HT and KO mice compared with WT mice. GAPDH was used as a loading control. A1, Quantification analysis of expression of Mfn‐2 protein levels when normalized with GAPDH, showing that Mfn‐2 levels increased significantly among WT, gas7 HT, and gas7 KO mice. A2, Quantification analysis of expression of Mfn‐1 protein levels when normalized with GAPDH showing Mfn‐1 levels increased significantly among WT, gas7 HT, and gas7 KO mice. A3, Quantification analysis of the phosphorylation status of Mfn‐2 protein levels (S442) when normalized with nonphosphorylated Mfn‐2 showing that Mfn‐2 phosphorylation decreased significantly among WT, gas7 HT, and gas7 KO mice. B, Western blot analysis. Total brain lysates from WT, gas7 HT, and gas7 KO mice were electrophoresed by 10% SDS‐PAGE. After blotting, the membranes were probed with Gas7 antibody to show that Gas7 protein exists in wild‐type and gas7 heterozygotes, but not in gas7‐knockout mice. Blots to detect expression of Drp‐1, GAPDH, Gas7, Fis‐1, phosho‐Drp‐1(S616), phosho‐Drp‐1(S637), phospho‐CREB(S133), CREB, CDK‐5, and GAPDH from cerebrum lysates of Gas7 WT, HT, and KO mice show decreased phospho‐Drp‐1(S616) (quantified in B2) and increases in both phospho‐Drp‐1 (S637) (quantified in B1) and phospho‐CREB in HT and KO mice compared with WT mice. There were no significant changes in Drp‐1, Fis‐1, CDK‐5 (quantified in B3), or CREB protein levels. GAPDH was used as a loading control
