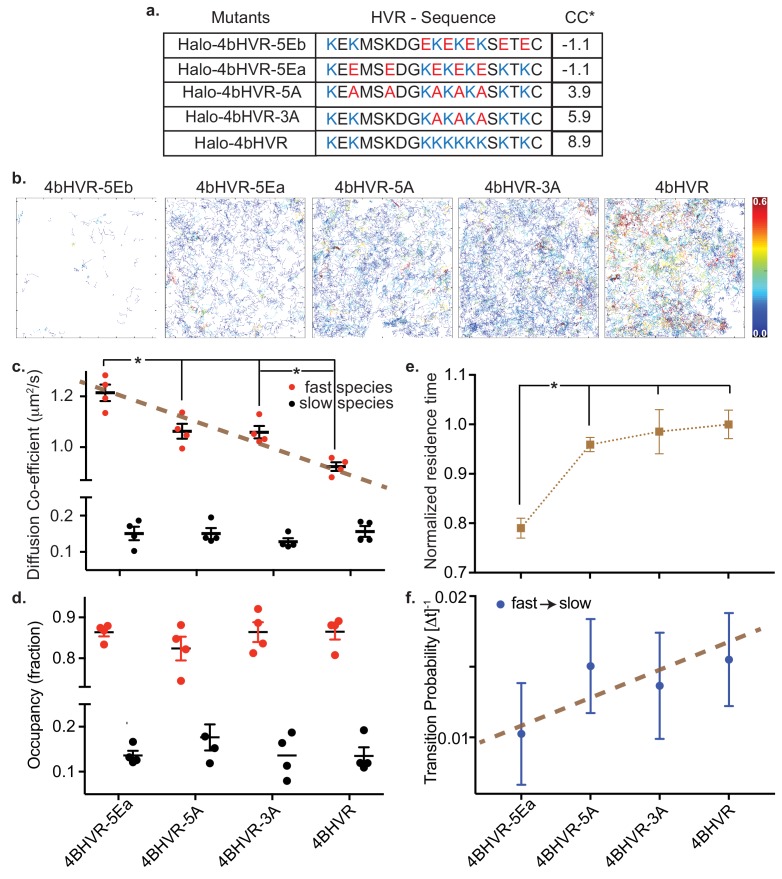
Figure 3. HMM analysis of KRAS4b HVR and mutations that adjusted the charges of the HVR.
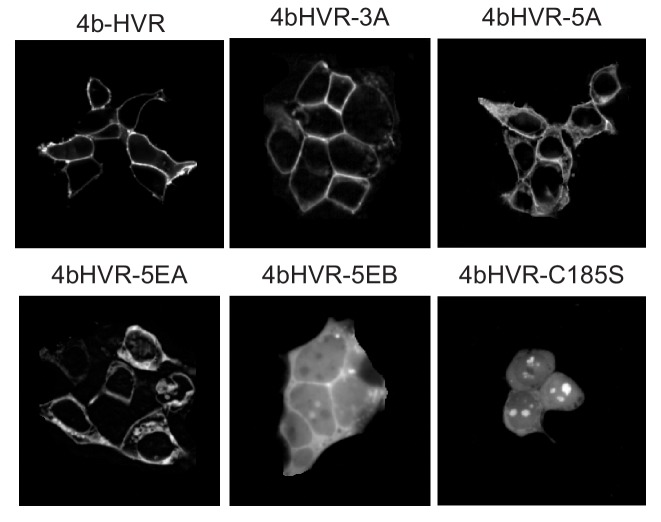
(a) Table shows HaloTag HVR (of KRAS4b) constructs over-expressed in HeLa cells by transient transfection with charge-neutral and charge-reversed substitution mutations of lysine residues with varying net charge content (*CC). (b) Single molecule tracks are shown for WT HVR and each of the mutant HVRs, color-coded according to their residence time at the membrane. The color bar encodes time from 0.0 s to 0.6 s. (c and d) Diffusion coefficient and occupancy of the fast and slow diffusing species are plotted in red and black solid circles respectively for each charge-altered mutant. While the fast diffusing species exhibit a gradual, significant (p<0.05) increase in diffusion coefficient as the lysine charges are neutralized and then reversed, the slow species remain the same and the relative fraction of fast and slow diffusing species remain unchanged. (e) Normalized average residence time from more than 5000 tracks for each mutant is shown in the plot. Significant reduction (p<0.05) in residence time indicates impaired association between charge-reversed HVR and membrane. (f) Graph shows transition probabilities from fast to slow diffusive state for each charge-altered mutant.