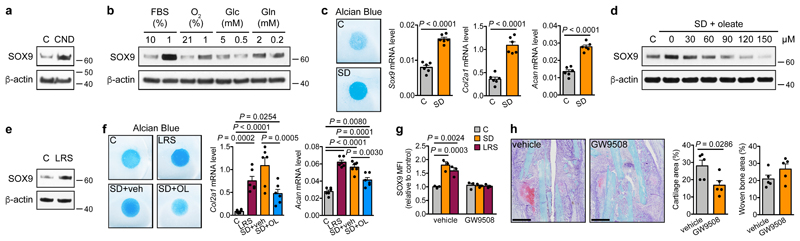
Figure 2. Lipid scarcity induces SOX9 in skeletal progenitors.
(a,b) Immunoblot detection of total SOX9 in C3H10T1/2 cells exposed for 24 hours to control or CND medium (a) or to different nutritional stresses (b), with β-actin as loading control (n=2 independent experiments). (c) Chondrogenic differentiation of periosteal cells in control or SD medium, assessed by visualization of chondrogenic matrix deposition (Alcian Blue staining) and quantification of Sox9, Col2a1 and Acan mRNA levels (relative to Actin, n=6 biologically independent samples). (d,e) Immunoblot detection of total SOX9 in C3H10T1/2 cells exposed for 6 hours to control, SD or SD medium supplemented with increasing concentrations of oleate (d) or to LRS medium (e), with β-actin as loading control (n=2 independent experiments). (f) Chondrogenic differentiation of periosteal cells in control, LRS, SD or SD medium supplemented with 60μM oleate (OL), assessed by Alcian Blue staining and quantification of Col2a1 and Acan mRNA levels (relative to Actin, n=6 biologically independent samples). (g) Flow cytometric quantification of total SOX9 levels in periosteal cells exposed for 24 hours to control, SD or LRS medium supplemented with 100μM GW9508 (FFAR1/4 agonist) or vehicle (DMSO) (n=3 biologically independent samples). (h) Histological visualization (Safranin O staining) and quantification of cartilage and woven bone in the callus at PFD7 of mice treated daily with GW9508 (10nmol) or vehicle (0.2% DMSO in saline) at the fracture site (n=5 mice). Scale bars, 500μm. Mean ± s.e.m. Two-tailed Student’s t-test (c,h), one-way ANOVA (f) or two-way ANOVA (g) with Bonferroni post-hoc test. For gel source data, see Supplementary Figure 1.