Abstract
Background
The aim of this study was to reveal the correlations between serum concentration of Clara cell secretory protein (CC16) and clinical parameters of stable chronic obstructive pulmonary disease (COPD).
Patients and Methods
Serum concentration of CC16 was determined by enzyme-linked immunosorbent assay (ELISA). The correlations between serum concentration of CC16 and clinical parameters was performed by linear correlation analysis and multiple linear regression analysis. The sensitivity and specificity of serum CC16 for differential diagnosis of COPD were determined by receiver operator characteristic curve (ROC).
Results
The serum concentration of CC16 was down-regulated in stable COPD patients compared with healthy control group (p < 0.05). The decreased serum CC16 was negatively related to smoking (p < 0.05), GOLD grading (p < 0.005), mMRC score (p < 0.05) and medical history (p < 0.05) of patients, but positively correlated with pulmonary function (p < 0.05). The smoking, FEV1/FVC values, COPD grading and mMRC scores all affected the concentration of CC16 (p < 0.05). The decreased CC16 was an independent risk factor in the process of deterioration of lung function. The sensitivity and specificity of serum CC16 for identifying COPD reached to 65.3% and 75%.
Conclusion
Decreased serum concentration of CC16 correlated with the disease progression of COPD, suggesting that it can be used as an indicator contributing to the diagnosis and assessment of COPD.
Keywords: chronic obstructive pulmonary disease, COPD, Clara cell secretory protein 16, CC16
Introduction
Chronic obstructive pulmonary disease (COPD) is a group of respiratory diseases characterized by chronic inflammation of the airways and incomplete reversible airflow limitation.1 According to statistics on the incidence and mortality of COPD in the United States, COPD is currently the fourth leading cause of death in the world and is expected to become the third leading cause of death in the world by 2020.1 Although the etiology and pathogenesis of COPD are not fully elucidated, airflow limitation is thought to be associated with chronic inflammation of the airways, which interacts and causes a progressive exacerbation of COPD.2 Clara cell secretory protein (CC16) is secreted by non-ciliated epithelial cells, Clara cells, on the bronchioles and terminal bronchioles, and has lung tissue specificity.3 As a homodimeric structure with a molecular weight of 15.8 kDa, CC16 has been found to play an important protective role in oxidative stress and inflammation of the respiratory tract,3 thus correlating with the development and progression of some lung diseases, including idiopathic pulmonary fibrosis, sarcoidosis, asthma and bronchiolitis obliterans.4
Currently, although the role of CC16 in the development of COPD has been initially confirmed, the association between the CC16 and COPD of different severity has not been elucidated. In this study, we tested the serum concentration of CC16 in patients with stable COPD and healthy individuals to provide a reference for the value of CC16 in diagnosis and disease assessment of COPD.
Methods
Statement for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)
The study consists of the following six parts: title, abstract, introduction, method, results, discussion and other information. The sample size and study variables were clearly described in this study. The statistical methods used in this study were explained in detail, and the systematic errors and data offsets of the variables were analyzed. The key findings of the study were carefully showed in the results and discussion. The keywords and terms used in the text made sure its correct indexing in electronic databases.
Ethics Statement
The study was approved by Research Ethics Committees of research institutes (First Affiliated Hospital, Xi’an Medical University, Xi’an, China; Jining NO.1 People’s Hospital, Jining, China; Shenmu Hospital, Shenmu, China; Zhangye Second People Hospital, Zhangye, China; Minle County People’s Hospital, Minle, China; Minqin County People’s Hospital, Minqin, China; Gansu Provincial Hospital, Lanzhou, ChinaGansu Provincial Hospital, Lanzhou, China). The study was carried out in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained in strict accordance with the approved ethical guidelines before the patient’s samples and data were collected.
Objects
Ninety-eight patients with stable COPD between January 2015 and January 2018 were selected as the observation group, including 56 males and 42 females, aged 44–77 years, with an average of 63.8 years (Patients recruited from an indicated Ethics statement center). In the same period, 48 healthy subjects were collected from the above centers as normal controls, including 19 males and 29 females, aged 46–76 years, with an average of 62.5 years. Clinical information is shown in Table 1. There were no significant differences in demographic data between the two groups (p > 0.05).
Table 1.
Clinical Information and General Data of Included Patients (98 Cases)
| Items | Classification | COPD Group (N, %) | Control Group (N, %) |
|---|---|---|---|
| Gender | |||
| Male | 56 (57.1) | 19 (39.6) | |
| Female | 42 (42.9) | 29 (60.4) | |
| Age (years) | |||
| <70 | 42 (42.9) | 28 (58.3) | |
| ≧70 | 56 (57.1) | 20 (41.7) | |
| Smoking | |||
| Yes | 46 (46.9) | 14 (29.2) | |
| No | 52 (53.1) | 34 (70.8) | |
| GOLD grade | |||
| 1 | 5 (5.1) | ||
| 2 | 19 (19.4) | ||
| 3 | 74 (75.5) | ||
| mMRC score | |||
| 2 | 12 (12.2) | ||
| 3 | 44 (44.9) | ||
| 4 | 42 (42.9) | ||
| Clinical history (years) | |||
| <10 | 31 (31.6) | ||
| ≧10 <20 | 28 (28.6) | ||
| ≧20 | 39 (39.8) |
Notes: GOLD grade: 1 = the forced expiratory volume in one second (FEV1) % predicted is more or equal to 80%, 2 = the FEV1% predicted is more or equal to 50%, but less than 80%, 3 = the FEV1% predicted is more or equal to 30%, but less than 50%, and 4 = the FEV1% predicted is less than 30%; mMRC, modified British Medical Research Council for dyspnea scale for symptom classification of COPD.
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, global initiative for chronic obstructive lung disease.
Diagnosis of COPD and Definition of Stable COPD
The COPD patients enrolled in this study met the diagnostic criteria of the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD):5,6 (1) symptoms of cough and dyspnea; (2) individual susceptibility factors and environmental factors; (3) after inhalation of 300 to 400 μg of salbutamol, the ratio of forced expiratory volume (FEV1) in the first second to forced vital capacity (FVC) is less than 0.70. Definition for stable COPD: the patient’s cough, expectoration and shortness of breath are in stable condition or just show mild symptoms, or the condition is basically restored to the state before acute exacerbation.7
Severity Rating of COPD
The severity grading of COPD is classified according to the grading standards of the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD):7,8 (1) GOLD-1, FEV1% predicted is more or equal to 80%; (2) GOLD-2, the FEV1% predicted is more or equal to 50%, but less than 80%; (3) GOLD-3, the FEV1% predicted is more or equal to 30%, but less than 50%; and (4) GOLD-4, the FEV1% predicted is less than 30%.
Inclusion Criteria
Inclusion criteria: (1) diagnosed as COPD and met the diagnostic criteria for stable COPD;7 (2) pulmonary function tests were performed within 7 days prior to enrollment; (3) no antibiotics, glucocorticoids and theophylline were treated within 2 weeks; (4) no purulent sputum and fever, no increase in white blood cell count or percentage of neutrophils, and no imaging evidence of pneumonia.
Exclusion Criteria
Exclusion criteria: (1) acute exacerbation of COPD; (2) with other lung diseases: asthma, interstitial lung disease, lung malignancy, pneumonia and tuberculosis; (3) diabetes, coronary heart disease, hypertension, cerebrovascular, nervous system diseases or liver and kidney dysfunction; (4) mental illness, cognitive dysfunction and communication disorders.
Specimen Collection
On the next day after the patient was enrolled, 5 mL of fasting elbow venous blood was taken in the morning, centrifuged (centrifugal radius 5 cm, rotation speed 3000 r/min) for 10 min, and the supernatant was taken and stored in a −70°C refrigerator.
Enzyme-Linked Immunosorbent Assay (ELISA)
The concentration of CC16 in serum was measured by sandwich-type ELISA (Nanjing Bosijin Biotechnology Co., Ltd, Nanjing, China) following the directions given by the manufacturer. The steps: (1) added 100 μL of the sample or standard to the reagent dilution and incubated for 2 hrs at 37°C; (2) added 100 μL of the detection antibody after washing and incubated at 37°C for 2 hrs; (3) added 100 μL of streptavidin-HRP working dilution after washing and incubated for 20 mins at 37°C in the dark; (4) added 100 μL of substrate solution after washing and incubated for 20 mins at 37°C in the dark; (5) added 50 μL of stop solution and mixed; (6) the optical density of each well was determined using a microplate reader set to 450 nm.
Modified British Medical Research Council (mMRC) Score
Dyspnea of patients was evaluated by the modified British medical research council (mMRC) scale which divided into 5 points.9 0 points: dyspnea will only occur after excessive activity; 1 point: dyspnea will occur after rapid walking; 2 points: dyspnea is more likely to occur during normal walking than normal people; 3 points: stop for breath after walking about a few minutes on level ground; 4 points: obvious breathing difficulties that affect normal life and work.
Evaluation of Objective Efficacy
Each patient was tested for lung function three times at different days, the average value was taken as statistical data. The statistical items selected in this study included FEV1/FVC and FEV1% predicted, which are directly related to the diagnosis and classification of COPD.
Statistical Processing of Research Data
The data of this study were analyzed by SPSS 21.0 software. The count data were expressed by [n (%)] for chi-square test. The measurement data were expressed as mean ± standard deviation (M±SD) and comparison between different groups was performed using the T test and one-way variance. The correlation analysis between the two factors was performed using a linear correlation analysis. The screening of risk factors was completed by multiple linear regression analysis. The sensitivity and specificity of serum indicator was figured out using receiver operating characteristic (ROC) curves. P < 0.05 means statistically significant.
Results
Concentration of Serum CC16 in Patients with Stable COPD Is Lower Than That in Healthy Controls
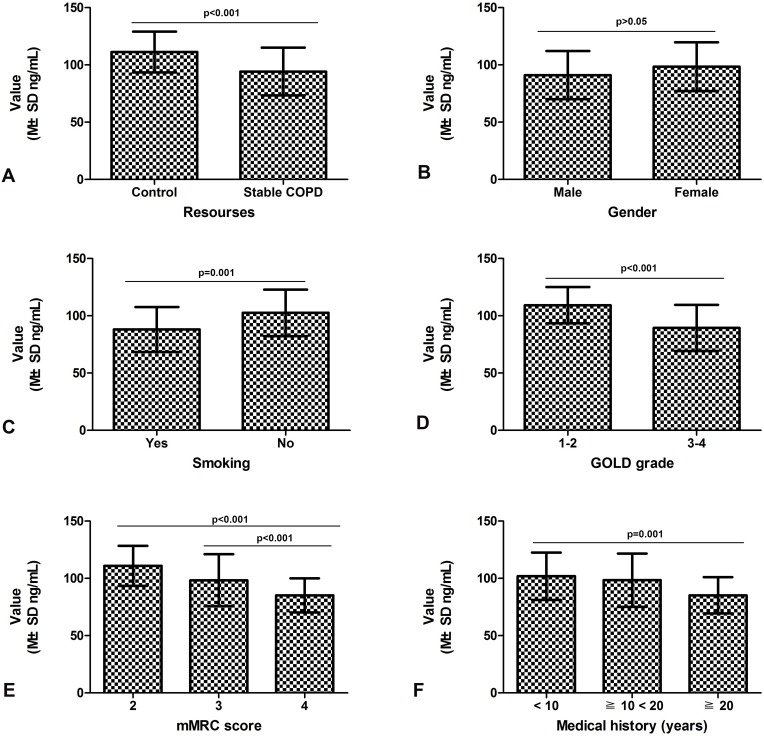
As shown in Table 2, the variance of serum CC16 value in stable COPD and healthy controls was equal (F = 1.265 and p = 0.263). The statistical results suggested that the concentration of serum CC16 in patients with stable COPD (94.2±20.9 ng/mL) was lower than that in healthy controls (111.3±17.9 ng/mL) (t = 4.839; df = 144; p < 0.001) (Figure 1A).
Table 2.
Relationship Between Clinical Parameters and the Serum Concentration of CC16 in Serum of Stable COPD Patients (n=98)
| Parameter | Group | N | Homogeneity Test of Variance | Level of CC16 in Serum of Stable COPD Patients | ||||
|---|---|---|---|---|---|---|---|---|
| F value | P value | Value (M±SD ng/mL) |
Degree of Freedom | Statistical Value | P value | |||
| Resources | ||||||||
| Control | 48 | 1.265 | 0.263 | 111.3±17.9 | 144 | 4.839 | <0.001 | |
| Stable COPD | 98 | 94.2±20.9 | ||||||
| Gender | ||||||||
| Male | 56 | 0.032 | 0.859 | 91.1±21.1 | 96 | −1.753 | 0.083 | |
| Female | 42 | 98.5±21.3 | ||||||
| Ages | ||||||||
| <70 | 46 | 0.015 | 0.902 | 97.3±20.3 | 96 | −1.386 | 0.090 | |
| ≧70 | 52 | 93.9±21.2 | ||||||
| Smoking | ||||||||
| Yes | 42 | <0.001 | 0.985 | 88.0±19.7 | 96 | 3.598 | 0.001 | |
| No | 56 | 102.5±20.3 | ||||||
| GOLD grade | ||||||||
| 1–2 | 24 | 3.295 | 0.073 | 109.2±15.8 | 96 | 4.382 | <0.001 | |
| 3–4 | 74 | 89.4±20.2 | ||||||
| mMRC score | ||||||||
| 2 | 12 | 2.156 | 0.081 | 110.9±17.5 | 2 | 10.136 | <0.001 | |
| 3 | 44 | 98.3±22.9★ | ||||||
| 4 | 42 | 85.2±14.9★★ | ||||||
| Medical history (years) | ||||||||
| <10 | 31 | 1.702 | 0.188 | 101.9±20.7 | 2 | 7.07 | 0.001 | |
| ≧10 <20 | 28 | 98.4±23.3 | ||||||
| ≧20 | 39 | 85.2±15.8★★★ | ||||||
Notes: ★The patients with mMRC score of 3 showed a lower expression level of serum CC16 than those with mMRC score of 2; ★★The patients with mMRC score of 4 showed a lower expression of serum CC16 than those with mMRC score of 2 and 3; ★★★The expression of serum CC16 in the patients with a longer history was down-regulated compared to those with relatively short history.
Abbreviations: N, number; COPD, chronic obstructive pulmonary disease; CC16, Clara cell secretory protein 16; M±SD, mean ± standard deviation; GOLD, global initiative for chronic obstructive lung disease; mMRC, modified British Medical Research Council.
Figure 1.
Relationship between clinical parameters and serum concentration of CC16 in stable COPD patients.
Notes: (A) Serum concentration of CC16 was higher in stable COPD patients than that in healthy control group (p < 0.001). (B) Serum concentration of CC16 was not related to the patient’s gender (p > 0.05). (C) Stable COPD patients with smoking showed an increased serum concentration of CC16 compared to the patients without smoking (p = 0.001). (D) Serum concentration of CC16 of patients of GOLD-3 and 4 was decreased compared with that of GOLD-1 and 2 (p < 0.001). (E) The patients with 4 points of mMRC scores showed a low serum concentration of CC16 compared with those with 2 and 3 points (p < 0.001). (F) Serum CC16 concentration in the patients with longer clinical history was decreased compared to those with short history (p = 0.001).
Abbreviations: COPD, chronic obstructive pulmonary disease; CC16, Clara cell secretory protein 16; M±SD, mean ± standard deviation; GOLD, global initiative for chronic obstructive lung disease; mMRC, modified British Medical Research Council.
Smoking Increases Concentration of Serum CC16 in Stable COPD Patients
As shown in Table 2, serum concentration of CC16 was not associated with gender in stable COPD patients (p > 0.05; Figure 1B). The variance of serum CC16 value in stable COPD with smoking and without smoking was equal (F < 0.001 and p = 0.985). The statistical results showed that the serum concentration of CC16 in stable COPD patients with smoking (88.0±19.7 ng/mL) was lower than that in patients without smoking (102.5±20.3 ng/mL) (p = 0.001; Figure 1C).
Serum CC16 Concentration in Patients with Stable COPD Is Negatively Correlated with the Patient’s GOLD Grade
As shown in Table 2, under the premise that the variance of the two groups was equal (F = 3.295 and p = 0.073), the statistical results showed that the serum concentration of CC16 in stable COPD patients at stage of GOLD-3 to 4 (89.4±20.2 ng/mL) was lower than that in patients at stage of GOLD-1 to 2 (109.2±15.8 ng/mL) (p < 0.001; Figure 1D).
Serum CC16 Concentration in Patients with Stable COPD Is Negatively Correlated with the Patient’s mMRC Score
As shown in Table 2, the variance of the data on the concentration of CC16 in mMRC score was also equal (F = 2.156 and p = 0.081). The statistical results showed that the serum concentration of CC16 in stable COPD patients with mMRC score of 3 (98.3±22.9 ng/mL) and 4 (85.2±14.9 pg/mL) was lower than that in patients with mMRC score of 2 (110.9±17.5 ng/mL) (p < 0.001; Figure 1E).
Serum CC16 Concentration in Patients with Stable COPD Is Negatively Correlated with the Patient’s Clinical Medical History
As shown in Table 2, the overall variance of the data on the serum concentration of CC16 in the serum of patients with stable COPD and the patient’s clinical history was equal (F = 1.702 and p = 0.188). The statistical results showed that the serum concentration of CC16 in stable COPD patients with long clinical history (85.2±15.8 ng/mL for ≧ 20 years) was lower than that in patients with short history (98.4±23.3 ng/mL for history ≧ 10 < 20 years; 101.9±20.7 ng/mL for history <10 years) (p = 0.001; Figure 1F).
Concentration of Serum CC16 in Patients with Stable COPD Is Positively Correlated with FEV1/FVC and FEV1% Predicted
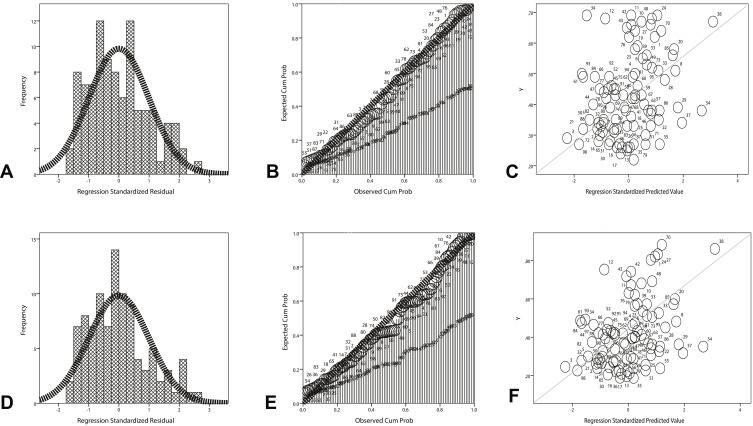
As shown in Table 3, the serum CC16 concentration was positively correlated with the FEV1/FVC (r = 0.387, p = 0.002; F = 8.588; t = 2.931, p = 0.004; the regression equation = ^Y=0.26+0.02X) (Figure 2A–C) and the FEV1% predicted (r = 0.361, p < 0.001; F = 14.4; t = 3.795, p < 0.001; the regression equation = ^Y=0.03+0.14X) (Figure 2D–F) in stable COPD patients.
Table 3.
Correlation Between Serum CC16 Concentration and Pulmonary Function in Patients with Stable COPD (n=98)
| Items | Correlation Analysis | ||
|---|---|---|---|
| CC16 and FEV1/FVC | CC16 and FEV1% Predicted | ||
| Pearson correlation | Correlation coefficient | 0.387 | 0.361 |
| P value | 0.002 | <0.001 | |
| R Square | 0.173 | 0.121 | |
| Analysis of Variance | Mean square | 0.335 | 0.356 |
| F value | 8.588 | 14.4 | |
| P value | 0.004 | <0.001 | |
| Regression coefficient | Regression coefficients | 0.02 | 0.14 |
| T value | 2.931 | 3.795 | |
| P value | 0.004 | <0.001 | |
| 95% confidence interval | 0.001–0.003% | 0.001–0.004% | |
| Equation | ^Y=0.26+0.02X | ^Y=0.03+0.14X | |
Abbreviations: CC16, Clara cell secretory protein; FEV1, the value of forced expiratory volume in one second; FVC, forced vital capacity.
Figure 2.
Linear regression analysis between serum concentration of CC16 and lung function test in stable COPD patients.
Notes: (A) The histogram of normal curve showed that the normalized residuals are normal distributions (p > 0.05). (B) The dependent variable is approximately linear with the standardized predictive value, indicating that CC16 concentration and FEV1/FVC have a linear correlation (p > 0.05). (C) The cumulative probability plot of the observations suggested that the two variables have a feature of normal distribution (p < 0.05). (D) The histogram of normal curve showed that the normalized residuals are normal distributions (p > 0.05). (E) The dependent variable is approximately linear with the standardized predictive value, indicating that CC16 concentration and FEV1% predicted have a linear correlation (p > 0.05). (F) The cumulative probability plot of the observations suggested that the two variables have a feature of normal distribution (p < 0.05).
Abbreviations: CC16, Clara cell secretory protein 16; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Smoking, High GOLD Grade and mMRC Score and Low FEV1/FVC are Risk Factors for Decreased Serum Concentration of CC16
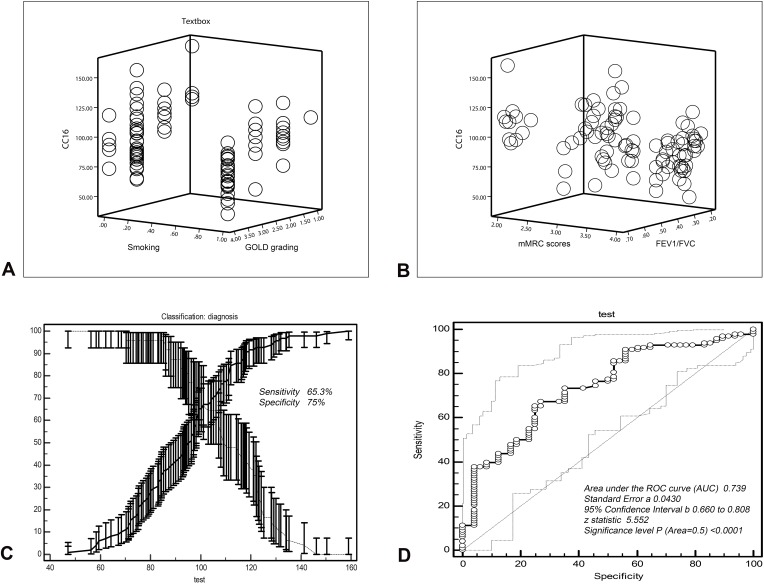
As shown in Table 4, smoking (p = 0.003; 95% CI = −1.057 to −0.182), high GOLD grade (p < 0.001; 95% CI = −32.582 to −16.063), high mMRC score (p = 0.006; 95% CI = −1.015 to −0.126) and the low FEV1/FVC (p < 0.001; 95% CI = −173.562 to −56.131) seemed to affect the serum concentration of CC16 in patients with stable COPD (the regression equation = ^Y=28.246–5.710X1-25.823 X2-8.976X3-114.431X4). Subgroup analysis showed that FEV1/FVC (partial regression analysis = −114.431) had a greater effect on CC16 concentration than the other three indicators (partial regression analysis = −5.710 for smoking; −25.823 for GOLD grading and −8.976 for mMRC scores) (Figure 3A and B).
Table 4.
Multiple Linear Regression Analysis to Determine the Factors That Affect the Serum Concentration of CC16 in Stable COPD (n=98)
| Items | Multiple Linear Regression Analysis for CC16 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unstandardized Coefficients | Variance Analysis | Standardized Coefficients | T value | P value | 95% Confidence Interval for B | ||||
| Partial Regression Analysis (B) | Std. Error | F value | P value | Beta value | Lower Bound | Upper Bound | |||
| Smoking (X1) | −5.710 | 2.246 | 29.528 | <0.001 | −0.263 | −2.546 | 0.003 | −1.057 | −0.182 |
| GOLD grading (X2) | −25.823 | 4. 915 | −1.046 | −5.253 | <0.001 | −35.582 | −16.063 | ||
| mMRC scores (X3) | −8.976 | 3.174 | −0.292 | −2.828 | 0.006 | −1.015 | −0.126 | ||
| FEV1/FVC (X4) | −114.431 | 30.365 | −0.713 | −3.769 | <0.001 | −173.562 | −56.131 | ||
| Regression equation | ^Y=28.246–5.710X1-25.823 X2-8.976X3-114.431X4 | ||||||||
Abbreviations: GOLD, global initiative for chronic obstructive lung disease; mMRC, modified British Medical Research Council for dyspnea scale for symptom classification of COPDL; CC16, Clara cell secretory protein; FEV1, the value of forced expiratory volume in one second; FVC, forced vital capacity.
Figure 3.
Analysis of factors influencing serum CC16 concentration in patients with COPD and diagnostic value analysis.
Notes: (A) The patient’s status of smoking and COPD grading affected the serum CC16 concentration in patients with COPD (p < 0.05). (B) The patient’s mMRC score and FEV1/FVC affected the serum CC16 concentration in patients with COPD (p < 0.05) and FEV1/FVC has the most significant impact on CC16. (C) Selection of critical value (99.32 ng/mL) of serum CC16 concentration for discerning COPD patients from healthy individuals responded a sensitivity of 65.3% and specificity of 75.5%. (D) ROC curve of CC16 concentration for distinguishing COPD patients from healthy individuals (AUC=0.739).
Abbreviations: GOLD, global initiative for chronic obstructive lung disease; mMRC, modified British Medical Research Council; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; ROC, receiver operating characteristic curve; AUC, area under the curve.
Determination of Serum CC16 Concentration Contributes to the Differential Diagnosis of COPD
As shown in Table 5, the results of ROC curve analysis showed that the threshold for differentiating COPD patients from healthy individuals was 99.32 ng/mL. The sensitivity derived from this threshold was 65.3% and the specificity was 75%. The area under the curve (AUC) was 0.739 and the standard error was 0.043 (95% confidence interval = 0.66–0.808; Z value = 5.552; p < 0.0001) (Figure 3D).
Table 5.
Criterion Values and Coordinates of the ROC Curve of Serum Concentration of CC16 for Distinguishing Between COPD and Healthy People
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | 95% CI | −LR | 95% CI |
|---|---|---|---|---|---|---|---|---|
| ≤70.22 | 11.22 | 5.7–19.2 | 100.00 | 92.6–100.0 | 0.89 | |||
| ≤85.21 | 37.76 | 28.2–48.1 | 95.83 | 85.7–99.5 | 9.06 | 7.0–11.8 | 0.65 | 0.2–2.5 |
| ≤88.12 | 39.80 | 30.0–50.2 | 87.50 | 74.8–95.3 | 3.18 | 2.4–4.2 | 0.69 | 0.3–1.5 |
| ≤93.56 | 47.96 | 37.8–58.3 | 83.33 | 69.8–92.5 | 2.88 | 2.3–3.7 | 0.62 | 0.3–1.2 |
| ≤97.12 | 55.10 | 44.7–65.2 | 77.08 | 62.7–88.0 | 2.40 | 1.9–3.0 | 0.58 | 0.3–1.0 |
| ≤99.32* | 65.31 | 55.0–74.6 | 75.00 | 60.4–86.4 | 2.61 | 2.1–3.2 | 0.46 | 0.3–0.8 |
| ≤103.11 | 67.35 | 57.1–76.5 | 64.58 | 49.5–77.8 | 1.90 | 1.5–2.4 | 0.51 | 0.3–0.8 |
| ≤107.22 | 73.47 | 63.6–81.9 | 64.58 | 49.5–77.8 | 2.07 | 1.6–2.6 | 0.41 | 0.2–0.7 |
| ≤114.25 | 85.71 | 77.2–92.0 | 47.92 | 33.3–62.8 | 1.65 | 1.2–2.2 | 0.30 | 0.2–0.5 |
| ≤125.12 | 92.86 | 85.8–97.1 | 16.67 | 7.5–30.2 | 1.11 | 0.6–2.1 | 0.43 | 0.2–0.9 |
| ≤135.22 | 97.96 | 92.8–99.8 | 6.25 | 1.3–17.2 | 1.04 | 0.3–3.1 | 0.33 | 0.08–1.3 |
| ≤159 | 100.00 | 96.3–100.0 | 0.00 | 0.0–7.4 | 1.00 |
Note:* The threshold for differentiating COPD patients from healthy individuals.
Abbreviations: ROC, receiver operating characteristic curve; CC16, Clara cell secretory protein; COPD, chronic obstructive pulmonary disease; 95% CI, 95% confidence; +LR, positive likelihood ratio; −LR, negative likelihood ratio.
Discussion
Since there is a concentration gradient difference of CC16 in between the alveolar epithelial cell and peripheral blood, CC16 can be passively diffused into the serum through the pulmonary blood barrier. And the content of CC16 in extrapulmonary organs is about 20 times lower than that of lung tissue, so CC16 in serum is almost entirely from lung tissue.10 The destruction of tracheal epithelial cells and pulmonary blood barrier integrity may cause changes in serum CC16 concentration.3,11 The purpose of our study was to investigate the role and clinical significance of serum CC16 in patients with stable COPD. We found that CC16 concentration was significantly reduced in serum from patients with stable COPD compared with healthy controls, which reflects that CC16 may play a protective role in the development of COPD. Impaired pulmonary blood barrier caused by acute lung injury can lead to a transient increase in serum CC16 but airway epithelium damage caused by chronic lung injury can reduce the number of Clara cells and thus result in a persistent decline of CC16.12 So, the decrease in the number of Clara cells and CC16 secretion is the theoretical basis for the association of COPD with CC16, and the change in serum CC16 concentration is earlier than that of the lung function.13
Smoking is one of the major risk factors for COPD, and Clara cells may be one of the major targets attacked by toxic substances in cigarette smoke. Our study showed that stable COPD patients who smoked had significantly lower serum concentration of CC16 than those who did not, which suggests that the smoke toxins of cigarettes do affect the secretory function of Clara cells. Cigarette smoke can stimulate the body to produce a large number of oxygen-free radicals, activate oxidative stress cell pathways, induce DNA damage, and thereby damage airway Clara epithelial cells.14 It has been reported that the protein level of CC16 in the lung tissue of COPD patients with smoking shows a decreasing trend14 compared with non-smoking COPD.15 The lung function test can be used to determine the degree of airflow limit in patients with COPD, our study showed that the serum concentration of CC16 in stable COPD patients at stage of GOLD-3 to 4 was lower than that in patients at stage of GOLD-1 to 2, indicating that the serum CC16 concentration can reflect the severity of COPD. Previous evidence suggests that serum CC16 concentration is negatively correlated with the rate of decline in FEV1 and is associated with progression of COPD.16 It is believed that a decrease in serum CC16 concentration can be used as a biomarker for predicting lung function decline and disease progression in COPD patients.17
Subjective respiratory function in COPD patients shows practical value in the evaluation of COPD disease progression. It has been proven that the mMRC rating scale is of great value in assessing the severity of COPD, evaluating treatment outcomes, and predicting survival.9 Our data showed that the serum concentration of CC16 in stable COPD patients with mMRC score of 3 and 4 was lower than that in patients with mMRC score of 2, which means that serum CC16 can be used as an indicator to evaluate and predict changes in respiratory function in patients with COPD. A study suggests that serum CC16 is inversely related to the rate of decline in FEV1, and that low level of CC16 is associated with velocity of decline in lung function and progressive elevation of airflow limitation.18 We also found that the medical history of COPD patients was negatively correlated with the serum CC16 concentration, which reflects a causal relationship between the process of increasing COPD and the reduction of secretion of CC16. It is reported that CC16 can inhibit the migration of peripheral blood inflammatory cells and reduce the release of related inflammatory mediators such as prostaglandins, thromboxane A2, leukotrienes and platelet-activating factor, thereby reducing lung inflammation and damage.19
Through multiple linear regression analysis, we found that smoking, low pulmonary function and high mMRC score all affected the serum concentration of CC16, indicating that CC16 is a crucial protective factor to patients with COPD. Studies show that CC16 includes serine and thiol-containing protein structures that stabilize the metabolism of ciliated cells by binding to glycoproteins on the surface of airway ciliated epithelial cells to maintain their cleansing effect on the respiratory tract.3,4,18 To date, there is still a lack of reliable serological indicators for the assessment of stable COPD, the assessment of COPD is mainly based on changes in patient’s symptoms and lung function, but these changes usually lag behind actual pathological changes. CC16 has been found as a protective factor on the airway through mechanisms such as anti-oxidation and anti–inflammatory.10,13,19 In our study, the ROC curve showed that the sensitivity and specificity of CC16 serum concentration for differentiating COPD patients from healthy individuals were 65.3% and 75%, respectively, the area under the curve reached to 0.739, suggesting that CC16 can effectively reflect the changes of COPD and has a good value for screening of COPD.
In conclusion, serum CC16 is decreased in the stable COPD patients and decreased CC16 negatively correlates with smoking, GOLD grading, mMRC score and clinical history of patients but positively correlates with the pulmonary function. The serum CC16 has a good sensitivity and specificity in identifying COPD, suggesting that it contributes to the diagnosis and assessment of COPD. However, this study was only conducted for patients with COPD in Chinese medical institutions. The conclusions of this study may not necessarily reflect the relationship between the serum expression of CC16 and its clinical features in patients in other countries, especially different races. Therefore, in the future, the national regions and ethnic groups of patients should be expanded to further confirm the relationship between CC16 and COPD.
Acknowledgments
This study was supported by grants from Xi’an Medical University Doctoral Research Startup Funding.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Biener AI, Decker SL, Rohde F. Prevalence and treatment of chronic obstructive pulmonary disease (COPD) in the United States. JAMA. 2019;322(7):602. doi: 10.1001/jama.2019.10241 [DOI] [PubMed] [Google Scholar]
- 2.Biaoxue R, Liu Y, Li M, Fu T, Gao W, Liu H. Correlation of serum levels of HIF-1alpha and IL-19 with the disease progression of COPD: a retrospective study. Int J Chron Obstruct Pulmon Dis. 2018;13:3791–3803. doi: 10.2147/COPD.S177034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broeckaert F, Clippe A, Knoops B, Hermans C, Bernard A. Clara cell secretory protein (CC16): features as a peripheral lung biomarker. Ann N Y Acad Sci. 2000;923:68–77. doi: 10.1111/j.1749-6632.2000.tb05520.x [DOI] [PubMed] [Google Scholar]
- 4.Kropski JA, Fremont RD, Calfee CS, Ware LB. Clara cell protein (CC16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest. 2009;135(6):1440–1447. doi: 10.1378/chest.08-2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montes de Oca M, Lopez Varela MV, Laucho-Contreras ME, et al. Classification of patients with chronic obstructive pulmonary disease according to the Latin American Thoracic Association (ALAT) staging systems and the global initiative for chronic obstructive pulmonary disease (GOLD). Arch Bronconeumol. 2017;53(3):98–106. doi: 10.1016/j.arbres.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 6.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 7.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Respirology. 2017;22(3):575–601. [DOI] [PubMed] [Google Scholar]
- 8.Bellinger CR, Peters SP. Outpatient chronic obstructive pulmonary disease management: going for the GOLD. J Allergy Clin Immunol Pract. 2015;3(4):471–478; quiz 479–480. doi: 10.1016/j.jaip.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 9.Launois C, Barbe C, Bertin E, et al. The modified medical research council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med. 2012;12:61. doi: 10.1186/1471-2466-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAuley DF, Matthay MA. Clara cell protein CC16. A new lung epithelial biomarker for acute lung injury. Chest. 2009;135(6):1408–1410. doi: 10.1378/chest.09-0304 [DOI] [PubMed] [Google Scholar]
- 11.Mannino DM. Biomarkers for chronic obstructive pulmonary disease diagnosis and progression: insights, disappointments and promise. Curr Opin Pulm Med. 2019;25(2):144–149. doi: 10.1097/MCP.0000000000000549 [DOI] [PubMed] [Google Scholar]
- 12.Wutzler S, Lehnert T, Laurer H, et al. Circulating levels of Clara cell protein 16 but not surfactant protein D identify and quantify lung damage in patients with multiple injuries. J Trauma. 2011;71(2):E31–E36. [DOI] [PubMed] [Google Scholar]
- 13.Laucho-Contreras ME, Polverino F, Tesfaigzi Y, Pilon A, Celli BR, Owen CA. Club cell protein 16 (CC16) augmentation: a potential disease-modifying approach for chronic obstructive pulmonary disease (COPD). Expert Opin Ther Targets. 2016;20(7):869–883. doi: 10.1517/14728222.2016.1139084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu L, Di PY, Wu R, Pinkerton KE, Chen Y. Repression of CC16 by cigarette smoke (CS) exposure. PLoS One. 2015;10(1):e0116159. doi: 10.1371/journal.pone.0116159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lomas DA, Silverman EK, Edwards LD, Miller BE, Coxson HO, Tal-Singer R. Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax. 2008;63(12):1058–1063. doi: 10.1136/thx.2008.102574 [DOI] [PubMed] [Google Scholar]
- 16.Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. doi: 10.1056/NEJMoa1105482 [DOI] [PubMed] [Google Scholar]
- 17.Park HY, Churg A, Wright JL, et al. Club cell protein 16 and disease progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(12):1413–1419. doi: 10.1164/rccm.201305-0892OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerra S, Halonen M, Vasquez MM, et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med. 2015;3(8):613–620. doi: 10.1016/S2213-2600(15)00196-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laucho-Contreras ME, Polverino F, Gupta K, et al. Protective role for club cell secretory protein-16 (CC16) in the development of COPD. Eur Respir J. 2015;45(6):1544–1556. doi: 10.1183/09031936.00134214 [DOI] [PMC free article] [PubMed] [Google Scholar]