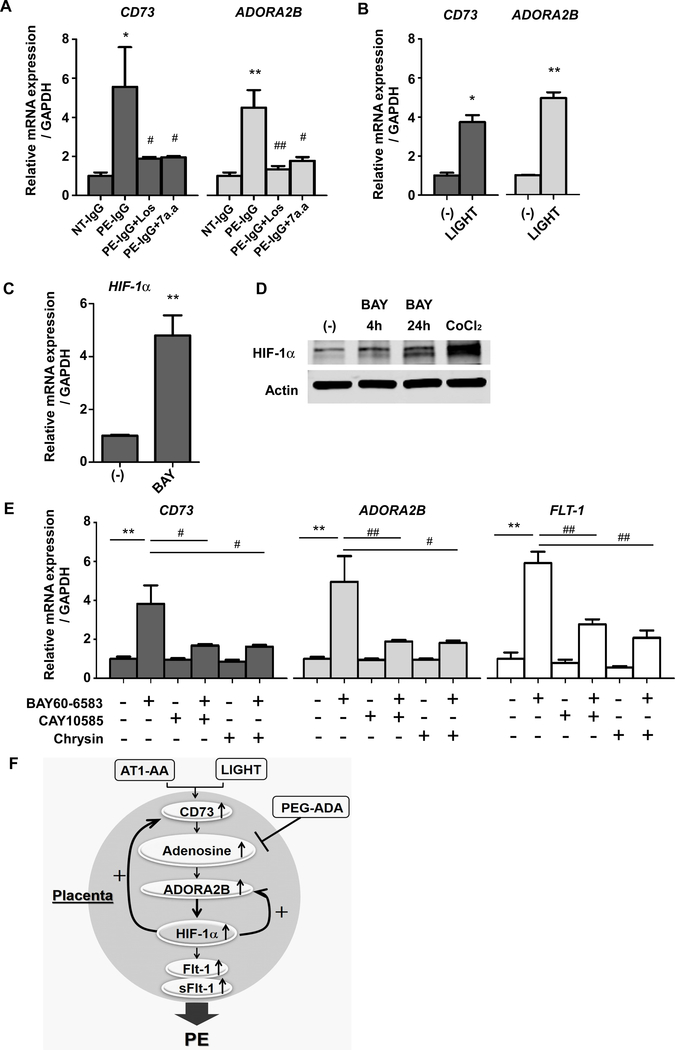
Figure 5. Upregulated HIF-1α via ADORA2B activation is responsible for the induction of CD73, ADORA2B, and FLT-1 gene expression in human villous explants.
(A) Human villous explants were treated with 100μg/ml NT-IgG or PE-IgG for 24 hours in the presence or absence of 5uM Losartan (Los) or 1uM autoantibody-neutralizing 7 amino acid epitope peptide (AFHYESQ, termed 7a.a). CD73 or ADORA2B mRNA levels were quantified using real-time RT-PCR. (n=3 independent experiments, *P<0.05, **P<0.01 vs NT-IgG, #P<0.05, ##P<0.01 vs PE-IgG)
(B) CD73 or ADORA2B mRNA levels in cultured human villous explants treated with 100pg/ml LIGHT or PBS for 24 hours were quantified using real-time RT-PCR. (n=3 independent experiments, *P<0.05, **P<0.01)
(C) HIF-1α mRNA levels in cultured human villous explants treated with ADORA2B agonist (1μM BAY60–6583) for 24 hours were quantified using real-time RT-PCR. (n=3 independent experiments, **P<0.01)
(D) HIF-1α protein determined by immunoblotting. Human villous explants were treated with 1μM BAY60–6583 for indicated hours or 100μM CoCl2 for 30 min as a positive control of HIF-1α production.
(E) Human villous explants were incubated for 24 hours and then pretreated with or without HIF-1α inhibitors (10μM CAY10585 or 1μM Chrysin) for 15 min and then treated with ADORA2B agonist (1μM BAY60–6583) for 24 hours. CD73, ADORA2B, and FLT-1 mRNA levels were determined by real-time RT-PCR. (n=3 independent experiments, **P<0.01, #P<0.05, ##P<0.01)
(F) Working model: The reinforcing reciprocal regulation of HIF-1α and adenosine signaling is the key driving force for their persistent elevation in the placenta. The malicious cycle of enhanced adenosine signaling in which HIF-1α plays a central role is a common causative factor underlying the pathophysiology of PE induced by AT1-AA or LIGHT.