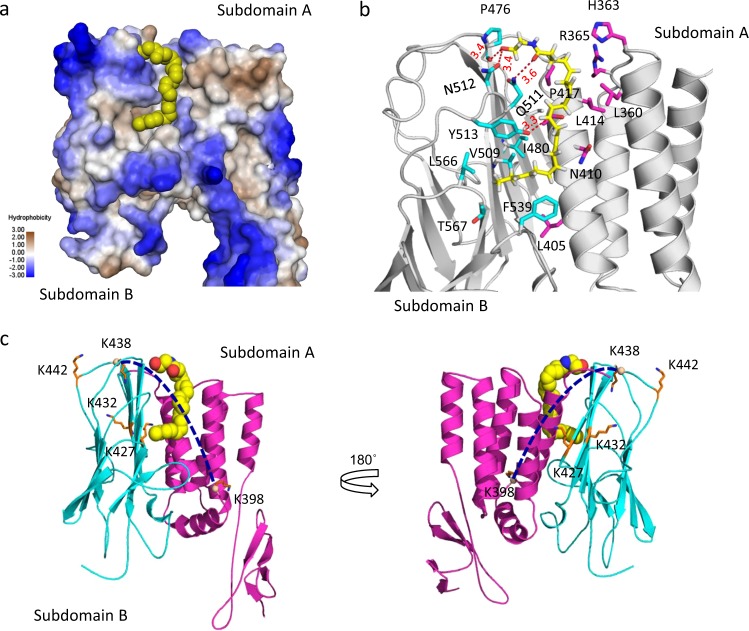
Fig. 4. Modeling of the GAIN domain interaction with synaptamide.
a Synaptamide binding pocket located in the interface of the two subdomains. The protein surface is colored with hydrophobicity (blue, polar; yellow-gray, hydrophobic). b Amino acid residues predicted to interact with synaptamide. c The inter-subdomain cross-linking of K398-K438 detected by in-cell cross-linking and mass spectrometry. Subdomains A and B are colored with magenta and cyan respectively. Among eight cross-linked lysine pairs observed, only the cross-link of K398-K438 (depicted by dotted line) changed after synaptamide binding. Lysin residues (stick representation) involving in the cross-linking reactions were labeled. The GAIN domain (AA 251–580) was modeled using the crystal structure of BAI3 (pdb 4DLO) as a template. Synaptamide is shown with a space-filling (a, c) or stick and ball representation (b).