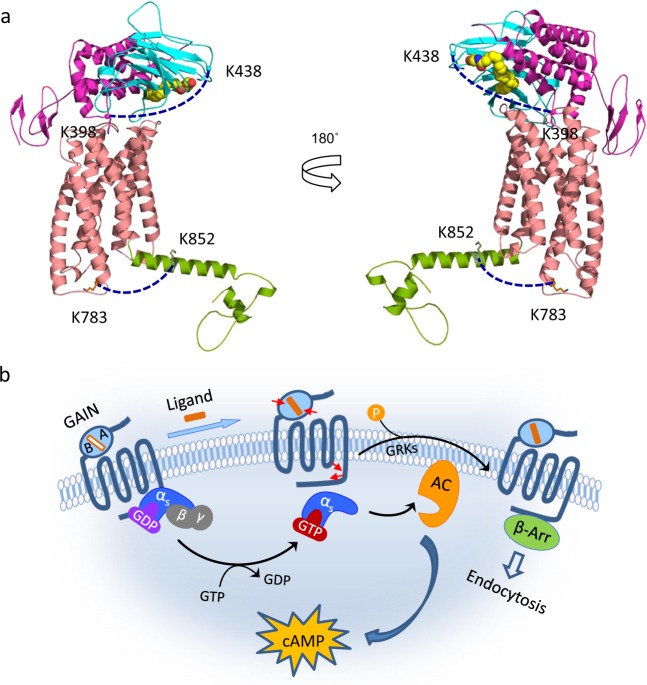
Fig. 6. A molecular mechanism of GPR110 activation.
a Ligand binding to the GAIN domain of the extracellular region induces conformational changes in GPR110. The conformational changes were deduced by monitoring the inter-subdomain cross-linking in the GAIN domain (K398-K438) and the intracellularly cross-linking between K783 of the TM6 and K852 of the C-terminal region (green). The K398-K438 and K783-K852 cross-links are indicated by dotted lines. The 7TM and C-terminal region of GPR110 were modeled with the structures of corticotropin-releasing factor receptor 1 (pdb 4K5Y) and glucagon receptor (pdb 4L6R). The GAIN domain was modeled using the crystal structure of BAI3 (pdb 4DLO). Synaptamide, the first small-molecule endogenous ligand for an aGPCR, is presented by a space-filling model. b A cartoon representation of GPR110 activation. Specific binding of synaptamide to the GAIN domain activates GPR110 through conformational changes (depicted by red arrows) within the GAIN domain and in the intracellular regions involving TM6 and the C-terminal tail. The intracellular conformational change results in Gs protein activation and β-arrestin recruitment. The ligand, and the binding pocket which is located at the interface between the subdomain A and subdomain B of the GAIN domain, are depicted with filled (orange) and open rectangles, respectively. AC Adenylyl cyclase, β-arr β-arrestin, GRKs G-protein-coupled receptor kinases.