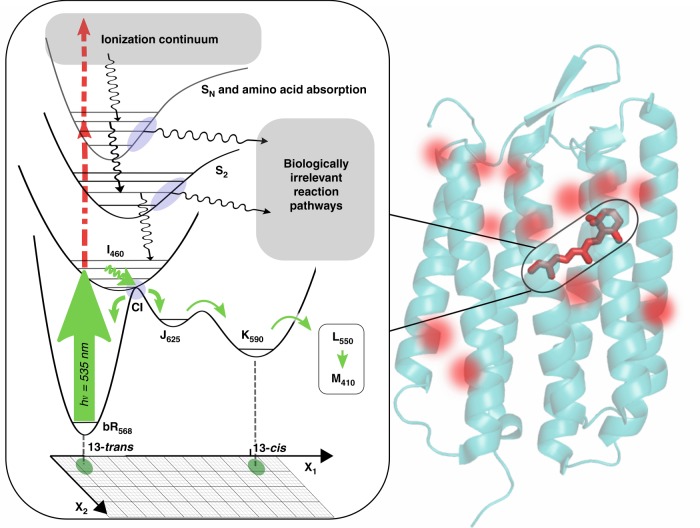
Fig. 2. Photophysical and chemical pathways at high peak power and fluences.
The seven transmembrane helical structure of bR is shown with retinal identified by the solid red structure. The red hot spots denote the positions for nonresonant two-photon excitation of Trp at high peak powers, which would be random and not contribute coherently to changes in diffraction (per ref. 3). However, all the resonant multiphoton pathways involving retinal would occur at the same location in space, i.e. at the retinal site, and contribute to photoinduced changes in diffraction. The left panel shows the biologically relevant one-photon pathway (solid green) for photoisomerization of retinal in relation to the multiphoton pathways (dashed green). The functionally relevant motions are highly constrained by the protein structure in going from the initially formed photoisomer, which is strongly coupled to distinct retinal-protein conformational states (I-M). In contrast, the different multiphoton pathways to different excited state surfaces have relaxation channels (black wiggly arrows) to photoproducts in undefined reaction coordinate space (x1, x2) unrelated to biological function. The need to excite the biologically relevant one-photon pathway is clear. This figure shows the importance of exciting in the one-photon, linear response, regime (see text for guidelines).