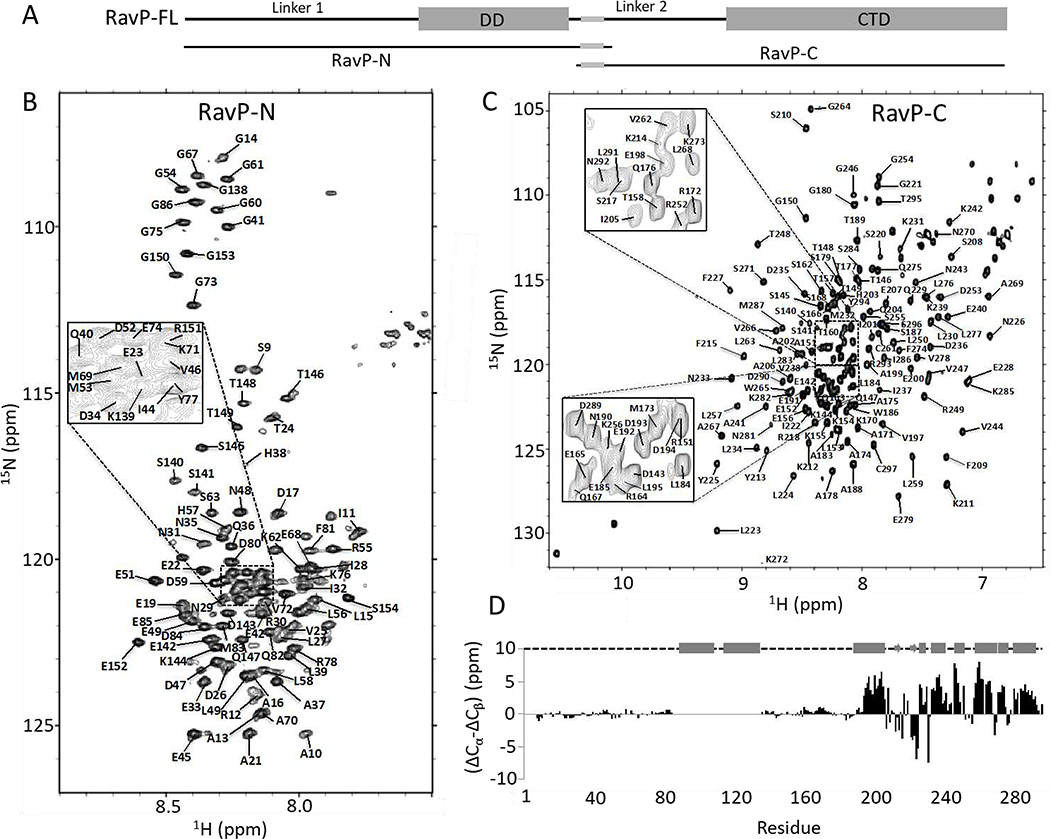
Fig. 2. Structural analysis and assignments of RavP constructs.
(A) Domain organization for RavP-N (1–152) and RavP-C(140–297) constructs. 15N,1H-HSQC spectra for RavP-N (B) and RavP-C (C) showing assignments. The spectra were acquired at 25 °C on a 700 MHz Bruker NMR spectrometer. (D) Secondary chemical shift values determined from Cα and Cβ shifts, showing two long disordered linkers and a well-ordered CTD, consisting of α-helices and β-sheets. The structural organization shown above was obtained from crystal structures of the CTD (PDB 1VYI) and dimerization domain (PDB 3L32), where cylinders represent α-helices; arrows represent β-strands, and dashed lines represent disordered regions.