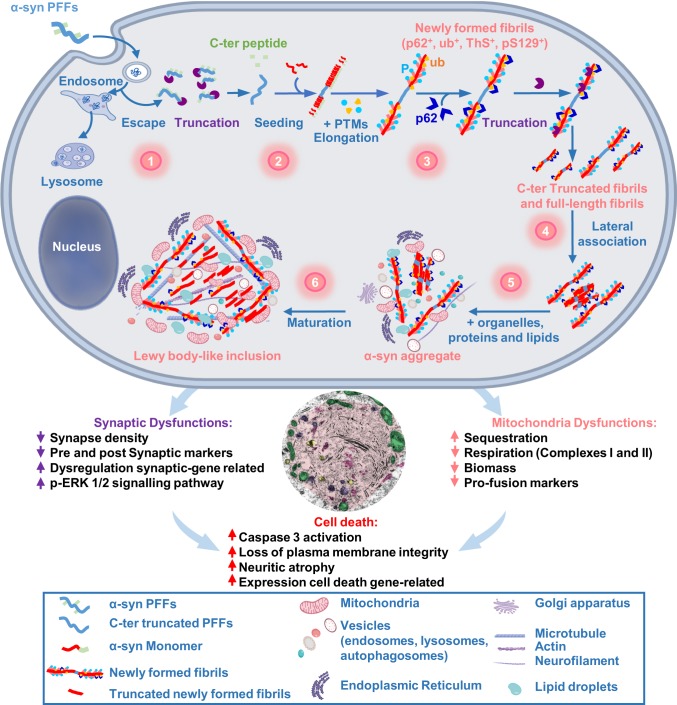
Fig. 8.
The dynamics of Lewy body formation, rather than simply α-syn fibrillization, is the primary cause of mitochondrial alterations, synaptic dysfunction, and neurodegeneration. Formation of α-syn inclusions in the context of the neuronal seeding requires a sequence of events starting with 1) the internalization and cleavage of PFF seeds (D0–D1); followed by 2) the initiation of the seeding by the recruitment of endogenous α-syn (D0–D4); 3) fibril elongation along with the incorporation of posttranslational modifications (PTMs), such as phosphorylation at residue S129 and ubiquitination (D4–D14); 4) formation of seeded filamentous aggregates (D7) that are fragmented and laterally associated over time (D7–D21); and 5–6) the packing of the fibrils into LB-like inclusions (D14–D21), which have a morphology similar to the bona fide LB detected in human synucleinopathies (D21), is accompanied by the sequestration of organelles, endomembranes, proteins, and lipids. Our integrated approach using advanced techniques in EM, proteomics, transcriptomics, and biochemistry clearly demonstrate that LB formation and maturation impaired the normal functions and biological processes in PFF-treated neurons, causing mitochondrial alterations and synaptic dysfunctions that result in a progressive neurodegeneration.