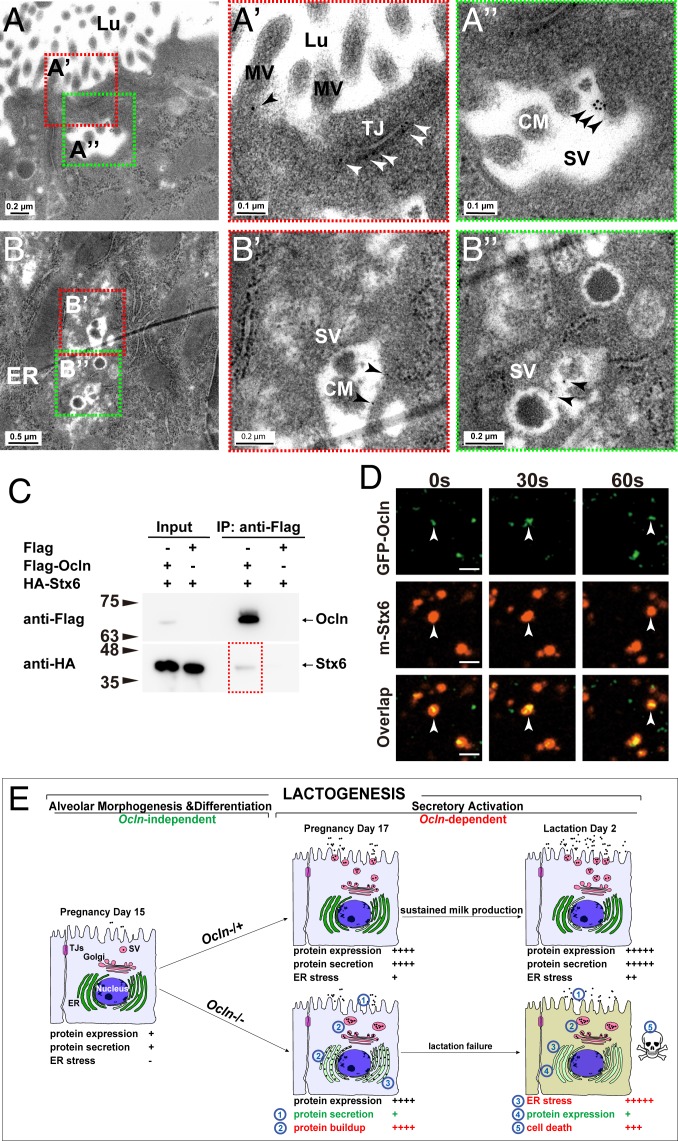
Fig. 7.
OCLN localized on SVs and bound to SNARE proteins. (A–B″) Localization of OCLN protein in luminal epithelial cells as detected by immunoelectron microscopy at the L2 stage. Primary antibodies against OCLN were visualized by a secondary antibody coupled to gold particles (black arrowheads). Overall, 19% of microvilli (out of a total of 304), 67% of TJs (out of a total of 12), and 40% of SVs (out of a total of 50) showed OCLN-positive staining. Areas in red or green dotted-boxes indicate close-up views on the right (A′, A″, B′, and B″). MV, microvillus; Lu, lumen; Nu, nucleus. Scale bars are as indicated. (C) Protein binding between OCLN and STX6 as detected by co-IP assays. OCLN was tagged by Flag protein, whereas STX6 was tagged by HA. Antibody against Flag was used for immunoprecipitation, and antibody against HA was used for subsequent Western blot analysis. Numbers indicate molecular weights of the markers (in kilodaltons). (D) Time course of localization of OCLN and STX6 as detected by fluorescent microscopy. GFP was fused in-frame with OCLN at the N terminus, whereas mCherry was fused in-frame with STX6. White arrowheads denote OCLN and STX6 particles over the time course of observation. Note that 20% of the OCLN particles colocalized with STX6 particles. (E) Schematic diagram of the lactogenic process, consisting of alveolar epithelial morphogenesis and differentiation, and secretory activation, which depends on Ocln function, in the mammary gland. In the Ocln−/+ control gland, there are progressive increases in milk protein expression and secretion, along with the accompanying stress buildup along the ER-Golgi secretory pathway during pregnancy and lactation. Ocln is essential for efficient protein secretion to meet the high demand of the greatly increased protein production and to relieve the resultant stress endured by the secretory pathway. As a result, copious amounts of milk are produced by Ocln control mammary glands to sustain pup growth. In mutant MPCs, loss of Ocln function results in defective protein secretion, which in turn causes a protein buildup along the ER-Golgi secretory pathway, including the ER and SVs. Consequently, ER stress and UPR are greatly increased, with an immediate response of shutdown of milk protein expression and, to a lesser extent, apoptosis of the MPCs that show long-term ER stress. As a result, milk production is greatly reduced, and Ocln mutant mice are unable to sustain pup survival.