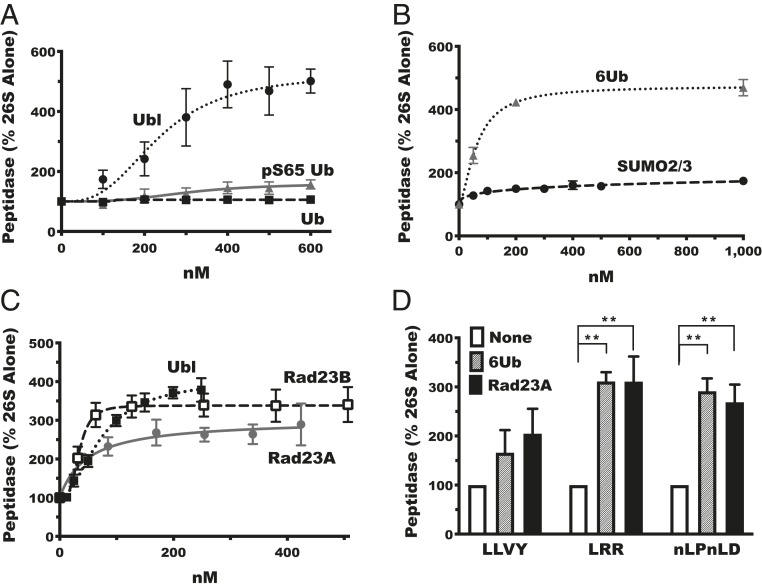
Fig. 1.
The shuttling factors Rad23A and Rad23B stimulate 26S proteasome peptidase activity. (A) The Rad23B Ubl domain (Ubl), but not monomeric ubiquitin (Ub) or phosphorylated monomeric ubiquitin (pS65 Ub), stimulates peptidase activity of purified rabbit muscle proteasomes measured by the hydrolysis of ac-Nle-Pro-Nle-Asp-amc. (B) SUMO2/3, unlike linear hexa-ubiquitin chains (6Ub), does not stimulate peptidase activity. (C) Full-length Rad23A and Rad23B and the Ubl domain of Rad23b (Ubl) stimulate peptidase activity similarly. (D) Linear chains of six ubiquitin (6Ub) and Rad23A stimulate the activity of all three peptidase activities of rabbit proteasomes: chymotrypsin-like activity measured with suc-Leu-Leu-Val-Tyr-amc (LLVY), trypsin-like activity measured with boc-Leu-Arg-Arg-amc (LRR), and caspase-like activity measured with ac-Nle-Pro-Nle-Asp-amc (nLPnLD). Error bars represent SEM (n = 3). **P < 0.01.