Abstract
Robotic surgery systems have been developed to overcome the limitations of laparoscopic surgery. Recently, Meerecompany Inc. in Korea successfully manufactured a robotic surgical system called Revo-i. A 65-year old woman was referred for a pancreatic head tumor, detected as an incidental finding during a routine check-up. Contrast abdominopelvic CT revealed a pancreatic uncinate tumor measuring around 13 mm in diameter, with no other focal lesions. The patient underwent a robot-assisted pancreaticoduodenectomy (laparoscopic resection and robotic reconstruction) using Revo-i. The patient's recovery was uneventful and discharged on postoperative day 7. Our case showed the technical feasibility of the Korean robotic surgical system Revo-i. Further experiences are mandatory to validate this finding.
Keywords: Robotic surgical system, Pancreaticoduodenectomy, Robot surgery
INTRODUCTION
Since the introduction of laparoscopic pancreaticoduodenectomy (LPD) in 1994 by Gagner and Pomp,1 many studies have demonstrated its safety and feasibility.2,3,4 However, owing to the technical complexity of the procedure, surgeons require sufficient training to develop skills for laparoscopic surgery.5,6
In general, PD comprises resection and reconstruction. Several issues are associated with the resection and reconstruction phases of LPD.7 Particularly, in benign and low-grade malignant tumors of the pancreas requiring PD, the remnant pancreas is associated with soft pancreas and small pancreatic ducts, which is the biggest risk factor for postoperative pancreatic fistula (POPF).8,9,10 Laparoscopic reconstruction of the remnant pancreas following the laparoscopic resection of the pancreaticoduodenal component is difficult. Skillful techniques and long surgical experience are required. Hence, a robotic surgical system may improve the surgical performance, safety, and effectiveness.
Robotic surgery has overcome the limitations of laparoscopic surgery.11 The advantages of robotic surgery, such as a minimized fulcrum effect, three-dimensional operative view, unrestricted instrument motion, and no involuntary tremors, enable surgeons to perform the surgery with ease and comfort.12,13 However, the high cost of a robotic surgical system is a great obstacle to its application in clinical practice, as only the commercial da Vinci Surgical System is available.
Fortunately, several robotic surgical systems have been developed to date.14,15,16,17 Recently, Meerecompany Inc. in Korea successfully manufactured a robotic surgical system called Revo-i. In recent studies, Chang et al.18 and Kang et al.19 performed robotic prostatectomy and cholecystectomy, respectively, using Revo-I, without any serious complications in the first human clinical trial. Finally, Revo-i was commercialized after obtaining the approval from the Korean Food and Drug Administration (KFDA).
Lately, we successfully conducted a robot-assisted minimally invasive PD (laparoscopic resection and robotic reconstruction) using Revo-i. Here, we report our first experience with Revo-i PD in a patient with insulinoma and discuss the current limitations and future of robotic surgery.
CASE
Case presentation
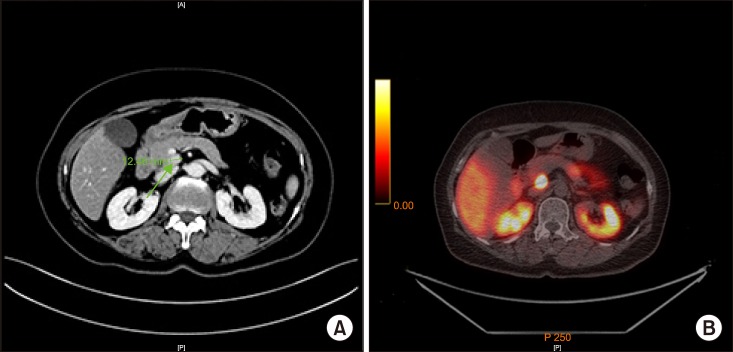
A 65-year-old woman was referred to our hospital for a pancreatic head tumor, detected as an incidental finding during a routine check-up. The patient had been treated for hypertension and had no family history of cancer. However, she reported of frequent neuroglycopenic symptoms during fasting conditions. There were no physical abnormalities, and her body mass index was 26.04 kg/m2. The laboratory data indicated a slight elevation of the level of insulin to 25.47 IU/ml (reference range: 1.0–10.7) and C-peptide to 4.60 ng/ml (reference range: 0.6–2.3), but no other abnormal findings. The tumor marker values were within the normal ranges: carcinoembryonic antigen 0.97 ng/ml and cancer antigen 19-9, 7.6 U/ml. Preoperative imaging (Fig. 1) revealed a pancreatic uncinate process tumor measuring 13 mm with no other significant focal lesions, suggesting a neuroendocrine tumor.
Fig. 1. Preoperative imaging. (A) Contrast abdominopelvic computed tomography revealed pancreatic uncinate tumor measuring around 13 mm in diameter, with no other focal lesions. (B) Positron emission tomography-computed tomography Ga-68 DODTATOC demonstrated significant focal intense DOTATOC uptake in the uncinate process of pancreas, suggesting neuroendocrine tumor.
Surgical procedure
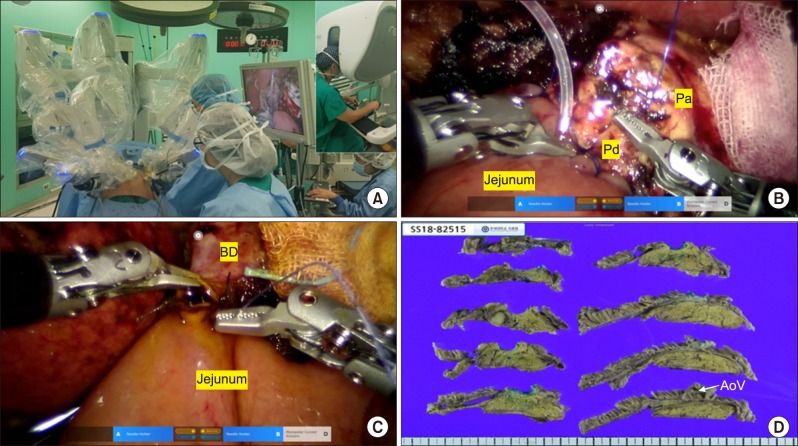
The patient underwent surgery on December 21, 2018. She was placed in a supine position. The surgery consisted of two processes: laparoscopic resection and robotic reconstruction. The laparoscopic resection technique used in our institution had been previously published.20 The total operation time for laparoscopic resection was 3 hours and 30 minutes, and the Revo-i robotic surgical system was docked (Fig. 2A). Revo-i-assisted pancreaticojejunostomy (PJ) with a double-layered duct-to-mucosa anastomosis (4 inner interrupted sutures for pancreatic duct less than 2 mm with a short stent and all interrupted sutures for the outer layer of PJ) (Fig. 2B) and choledochojejunostomy (CJ) (posterior continuous and anterior interrupted sutures using 4-0 absorbable sutures for 8 mm of remnant bile duct reconstruction) were performed (Fig. 2C), which took about 4 hours. The total operation time was noted to be 514 minutes. The estimated blood loss was 200 ml. No intraoperative transfusions were required. After the delivery of the specimen through the small, vertical, extended umbilical wound, duodenojejunostomy was performed manually. Two-armed closed suction drains were inserted around the anastomosis sites.
Fig. 2. Intraoperative and pathological findings. Pa, pancreas; Pd, pancreatic duct; BD, bile duct.
Postoperative course
The postoperative course was uneventful, except for mild fever. Her surgical drains were removed on postoperative day 5. A follow-up computed tomography (CT) was performed on postoperative day 7. Scant fluid along the PJ site was noted on CT. The patient was discharged 10 days after the operation, without any complications.
Pathological examination
The pathological diagnosis was neuroendocrine tumor (NET), measuring 11 mm in size (Fig. 2D). The mitotic count was 1 per 10 high-power fields, and the Ki-67 labeling index was 3/760 (0.4%). These results indicated Grade 1 NET based on the 2017 World Health Organization classification. No lymphovascular invasions were detected. A total of 6 lymph nodes were harvested, with no lymph node metastases. All the resection margins were negative.
DISCUSSION
The robotic surgery system has gained popularity because of various advantages over the laparoscopic surgery. It enables a more delicate and finer procedure. Furthermore, robotic surgeries show decreased postoperative morbidity and hospital stay and faster recovery of patients compared to the open approaches.21 They have now been widely applied in abdominal surgeries. According to the 2016 Intuitive Surgical Annual Report, there are over 3700 robotic surgical systems worldwide and over 3,000,000 robotic surgeries have been performed.22 Robotic pancreaticoduodenectomy is also feasible and safe in select patients.23,24,25 However, the high medical costs of robotic surgery, which have been an impediment to their development and evolution, have been constantly discussed.25,26,27 New robotic surgery systems with lower medical costs are required.
The Meerecompany Inc. has been developing a new robotic surgical system called Revo-i in Korea since 2006. Several preclinical studies have been conducted on robotic surgery using Revo-i in a porcine model.19,28,29,30 Kang et al.19 and Lim et al.30 simultaneously verified the safety and efficacy of robotic cholecystectomy performed using Revo-i in a preclinical experiment. Abdel Raheem et al.29 also showed the feasibility of fallopian tube surgery using Revo-i in a preclinical chronic porcine model. Owing to these results, the Revo-i received approval for clinical use from the KFDA in 2016. In the first clinical trial of Revo-i, Chang et al.18 successfully carried out 17 robotic surgeries on patients with localized prostate cancer, without any serious complications. In addition, Kang et al.19 performed successful Revo-i cholecystectomy in the first human clinical trial. Based on these positive perioperative outcomes in the human clinical trials of Revo-i, it was commercially licensed in August 2017.
In a previous study, the Revo-i characteristics were compared to the da Vinci Surgical System.30 The da Vinci Surgical System differed mainly with regard to energy sources. Currently, Revo-i has only mono-polar and bi-polar energy delivery systems. Therefore, Revo-i can be effectively used in the reconstruction phase of PD, but not in the resection phase, which requires fine dissection and rapid hemostasis. The next generation of Revo-i would be equipped with energy devices, such as vessel sealers and harmonic scalpels.
To the best of our knowledge, this is the first report of PPPD performed using Revo-I, which is an alternative robotic surgical system for the da Vinci Surgical System. In a previous human clinical trial of Revo-i cholecystectomy and prostatectomy, it was found technically feasible and safe in performing delicate and complex surgical procedures, such as PJ in remnant pancreas with a pancreatic duct shorter than 2 mm and CJ in a remnant bile duct smaller than 1 cm. The articulating movement of a robotic instrument and effective third-arm intervention are useful during the reconstruction phase of PD. However, for a safer and more reliable clinical application of Revo-i in advanced minimally invasive surgeries, improving the durability and fine tuning of robotic instruments and quality of vision should be mandatory. An additional clinical trial to investigate the safety of Revo-i in advanced minimally invasive hepatobiliary and pancreatic surgery should be performed.
In summary, we have successfully performed PD using Revo-i to treat a patient with insulinoma of the pancreatic uncinate process. This case demonstrated the technical feasibility of the Korean robotic surgical system Revo-i. We hope that other robotic systems would be developed to overcome the current limitations regarding cost effectiveness and to provide high-quality, minimally invasive surgeries in the near future.
ACKNOWLEDGEMENTS
The authors especially express their sincere thanks for the robotic surgery-specialized nurses, Hyun Jung Song, Soo Yeon Jung, and Soo Young Song in Severance Hospital, Seoul, KOREA, whose devotion and helpful comments during the operation enabled us to work on this procedure. Without their active support during Revo-i robotic surgical procedures, this surgery could not have been performed effectively.
Footnotes
CONFLICT OF INTEREST: Currently, the second clinical trial of Revo-i in advanced minimally invasive surgery is undergoing supported by meeraecompany research fund (research No. 1-2019-0030).
AUTHOR CONTRIBUTIONS: Kang I and Kang CM designed the study and drafted the manuscript. Kang I, Kang CM, Hwang HK, and Lee WJ participated in the acquisition of data, analysis, and interpretation of data.
References
- 1.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408–410. doi: 10.1007/BF00642443. [DOI] [PubMed] [Google Scholar]
- 2.Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg. 2010;145:19–23. doi: 10.1001/archsurg.2009.243. [DOI] [PubMed] [Google Scholar]
- 3.Boggi U, Amorese G, Vistoli F, Caniglia F, De Lio N, Perrone V, et al. Laparoscopic pancreaticoduodenectomy: a systematic literature review. Surg Endosc. 2015;29:9–23. doi: 10.1007/s00464-014-3670-z. [DOI] [PubMed] [Google Scholar]
- 4.Poves I, Burdío F, Morató O, Iglesias M, Radosevic A, Ilzarbe L, et al. Comparison of perioperative outcomes between laparoscopic and open approach for pancreatoduodenectomy: the PADULAP randomized controlled trial. Ann Surg. 2018;268:731–739. doi: 10.1097/SLA.0000000000002893. [DOI] [PubMed] [Google Scholar]
- 5.Nagakawa Y, Nakamura Y, Honda G, Gotoh Y, Ohtsuka T, Ban D, et al. Learning curve and surgical factors influencing the surgical outcomes during the initial experience with laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2018;25:498–507. doi: 10.1002/jhbp.586. [DOI] [PubMed] [Google Scholar]
- 6.Zhang T, Zhao ZM, Gao YX, Lau WY, Liu R. The learning curve for a surgeon in robot-assisted laparoscopic pancreaticoduodenectomy: a retrospective study in a high-volume pancreatic center. Surg Endosc. 2019;33:2927–2933. doi: 10.1007/s00464-018-6595-0. [DOI] [PubMed] [Google Scholar]
- 7.Kang CM, Lee SH, Chung MJ, Hwang HK, Lee WJ. Laparoscopic pancreatic reconstruction technique following laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2015;22:202–210. doi: 10.1002/jhbp.193. [DOI] [PubMed] [Google Scholar]
- 8.Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM., Jr Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg. 2007;245:443–451. doi: 10.1097/01.sla.0000251708.70219.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe YM, Lee KY, Oh CA, Lee JB, Choi SK, Hur YS, et al. Risk factors affecting pancreatic fistulas after pancreaticoduodenectomy. World J Gastroenterol. 2008;14:6970–6974. doi: 10.3748/wjg.14.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMillan MT, Malleo G, Bassi C, Sprys MH, Ecker BL, Drebin JA, et al. Pancreatic fistula risk for pancreatoduodenectomy: an international survey of surgeon perception. HPB (Oxford) 2017;19:515–524. doi: 10.1016/j.hpb.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Satava RM. Surgical robotics: the early chronicles: a personal historical perspective. Surg Laparosc Endosc Percutan Tech. 2002;12:6–16. doi: 10.1097/00129689-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Del Chiaro M, Segersvärd R. The state of the art of robotic pancreatectomy. Biomed Res Int. 2014;2014:920492. doi: 10.1155/2014/920492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stafford AT, Walsh RM. Robotic surgery of the pancreas: the current state of the art. J Surg Oncol. 2015;112:289–294. doi: 10.1002/jso.23952. [DOI] [PubMed] [Google Scholar]
- 14.Nio D, Bemelman WA, Busch OR, Vrouenraets BC, Gouma DJ. Robot-assisted laparoscopic cholecystectomy versus conventional laparoscopic cholecystectomy: a comparative study. Surg Endosc. 2004;18:379–382. doi: 10.1007/s00464-003-9133-6. [DOI] [PubMed] [Google Scholar]
- 15.Cadière GB, Himpens J, Germay O, Izizaw R, Degueldre M, Vandromme J, et al. Feasibility of robotic laparoscopic surgery:146 cases. World J Surg. 2001;25:1467–1477. doi: 10.1007/s00268-001-0132-2. [DOI] [PubMed] [Google Scholar]
- 16.Fanfani F, Monterossi G, Fagotti A, Rossitto C, Gueli Alletti S, Costantini B, et al. The new robotic TELELAP ALF-X in gynecological surgery: single-center experience. Surg Endosc. 2016;30:215–221. doi: 10.1007/s00464-015-4187-9. [DOI] [PubMed] [Google Scholar]
- 17.Yi B, Wang G, Li J, Jiang J, Son Z, Su H, et al. The first clinical use of domestically produced Chinese minimally invasive surgical robot system “Micro Hand S”. Surg Endosc. 2016;30:2649–2655. doi: 10.1007/s00464-015-4506-1. [DOI] [PubMed] [Google Scholar]
- 18.Chang KD, Abdel Raheem A, Choi YD, Chung BH, Rha KH. Retzius-sparing robot-assisted radical prostatectomy using the Revo-i robotic surgical system: surgical technique and results of the first human trial. BJU Int. 2018;122:441–448. doi: 10.1111/bju.14245. [DOI] [PubMed] [Google Scholar]
- 19.Kang CM, Chong JU, Lim JH, Park DW, Park SJ, Gim S, et al. Robotic cholecystectomy using the newly developed Korean robotic surgical system, Revo-i: a preclinical experiment in a porcine model. Yonsei Med J. 2017;58:1075–1077. doi: 10.3349/ymj.2017.58.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park H, Kang I, Kang CM. Laparoscopic pancreaticoduodenectomy with segmental resection of superior mesenteric vein-splenic vein-portal vein confluence in pancreatic head cancer: can it be a standard procedure? Ann Hepatobiliary Pancreat Surg. 2018;22:419–424. doi: 10.14701/ahbps.2018.22.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng L, Lin S, Li Y, Xiao W. Systematic review and meta-analysis of robotic versus open pancreaticoduodenectomy. Surg Endosc. 2017;31:3085–3097. doi: 10.1007/s00464-016-5371-2. [DOI] [PubMed] [Google Scholar]
- 22.Cole AP, Trinh QD, Sood A, Menon M. The rise of robotic surgery in the new millennium. J Urol. 2017;197(2S):S213–S215. doi: 10.1016/j.juro.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 23.Boggi U, Signori S, De Lio N, Perrone VG, Vistoli F, Belluomini M, et al. Feasibility of robotic pancreaticoduodenectomy. Br J Surg. 2013;100:917–925. doi: 10.1002/bjs.9135. [DOI] [PubMed] [Google Scholar]
- 24.Liao CH, Wu YT, Liu YY, Wang SY, Kang SC, Yeh CN, et al. Systemic review of the feasibility and advantage of minimally invasive pancreaticoduodenectomy. World J Surg. 2016;40:1218–1225. doi: 10.1007/s00268-016-3433-1. [DOI] [PubMed] [Google Scholar]
- 25.Kornaropoulos M, Moris D, Beal EW, Makris MC, Mitrousias A, Petrou A, et al. Total robotic pancreaticoduodenectomy: a systematic review of the literature. Surg Endosc. 2017;31:4382–4392. doi: 10.1007/s00464-017-5523-z. [DOI] [PubMed] [Google Scholar]
- 26.Tedesco G, Faggiano FC, Leo E, Derrico P, Ritrovato M. A comparative cost analysis of robotic-assisted surgery versus laparoscopic surgery and open surgery: the necessity of investing knowledgeably. Surg Endosc. 2016;30:5044–5051. doi: 10.1007/s00464-016-4852-7. [DOI] [PubMed] [Google Scholar]
- 27.Conlon KC, de Rooij T, van Hilst J, Abu Hidal M, Fleshman J, Talamonti M, et al. Minimally invasive pancreatic resections: cost and value perspectives. HPB (Oxford) 2017;19:225–233. doi: 10.1016/j.hpb.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Kim DK, Park DW, Rha KH. Robot-assisted partial nephrectomy with the REVO-I robot platform in porcine models. Eur Urol. 2016;69:541–542. doi: 10.1016/j.eururo.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 29.Abdel Raheem A, Troya IS, Kim DK, Kim SH, Won PD, Joon PS, et al. Robot-assisted Fallopian tube transection and anastomosis using the new REVO-I robotic surgical system: feasibility in a chronic porcine model. BJU Int. 2016;118:604–609. doi: 10.1111/bju.13517. [DOI] [PubMed] [Google Scholar]
- 30.Lim JH, Lee WJ, Park DW, Yea HJ, Kim SH, Kang CM. Robotic cholecystectomy using Revo-i Model MSR-5000, the newly developed Korean robotic surgical system: a preclinical study. Surg Endosc. 2017;31:3391–3397. doi: 10.1007/s00464-016-5357-0. [DOI] [PubMed] [Google Scholar]