Abstract
Background
Long-term administration of ethambutol (EMB) for Mycobacterium avium complex lung disease (MAC-LD) sometimes leads to permanent discontinuation of EMB due to various adverse events. This study aimed to investigate treatment outcomes after discontinuation of EMB.
Methods
Among patients diagnosed with MAC-LD between January 2001 and December 2014, 508 patients whose treatment was initiated with standard regimen until May 2018 were enrolled at a tertiary referral center in Korea. Of these 508 patients, 60 (11.8%) discontinued EMB due to various adverse effects. Among these 60 patients, treatment outcomes were analyzed for 44 patients by comparing their outcomes with those of matched subjects who received the standard treatment regimen without EMB discontinuation.
Results
The mean age of the 60 patients who discontinued EMB was 64.4 years. Ocular toxicity was the most common cause of discontinuation of EMB (75.0%, 45/60). The mean duration of EMB administration before its discontinuation was 7.0 ± 4.6 months. The treatment failure rate of the 44 patients with EMB discontinuation analyzed for treatment outcome was 29.6%, which was higher than that of the matched patients who received the standard regimen (18.3%), although the difference was not significant (P = 0.095). Of these 44 patients, EMB was substituted with later-generation fluoroquinolone in 23 patients, and the treatment failure rate of these 23 patients was significantly higher than that of the matched patients who received the standard regimen (39.1% vs. 19.3%, P = 0.045).
Conclusion
These findings suggest that treatment outcomes are unsatisfactory in patients with MAC-LD who discontinue EMB owing to adverse events. Notably, there was a statistically significant high failure rate in patients who were prescribed fluoroquinolone to replace EMB.
Keywords: Mycobacterium avium, Lung Disease, Ethambutol, Treatment Outcome, Fluoroquinolones
Graphical Abstract
INTRODUCTION
The prevalence and incidence of diseases caused by nontuberculosis mycobacterium (NTM) are increasing worldwide, including Korea.1 NTM constitutes diverse group of organisms such as Mycobacterium avium complex (MAC), the most frequently encountered NTM organism in many countries, including Korea.2 MAC lung disease (MAC-LD) is the most common clinical manifestation of MAC infection.3
The current guidelines for MAC-LD recommend a multidrug regimen comprising macrolide, ethambutol (EMB), and rifamycin with or without an aminoglycoside.3,4 The cornerstones of MAC-LD treatment are the macrolides, which include clarithromycin and azithromycin, but EMB is the second most important drug in the treatment of MAC-LD because it prevents the emergence of macrolide-resistant MAC isolates.3 However, long-term administration of EMB sometimes causes various adverse events such as ocular toxicity,5 cutaneous reaction, or cytopenia.6 These adverse events can result in inadvertent permanent discontinuation of EMB. However, in these circumstances it is unclear whether EMB should be substituted with other antibiotics or if treatment can be maintained with only the remaining drugs without the addition of a replacement for EMB. Because of the lack of guidance, attending physicians tend to choose their own treatment regimen under these circumstances. That is, an attending physician may either consider that EMB is an important companion drug and prescribe one or more other drugs as an alternative or continue treatment without adding other drugs to the existing treatment regimen. To the best of our knowledge, no studies concerning the outcome of these various treatments after discontinuation of EMB have been reported; therefore, we aimed to investigate this issue.
METHODS
Study subjects
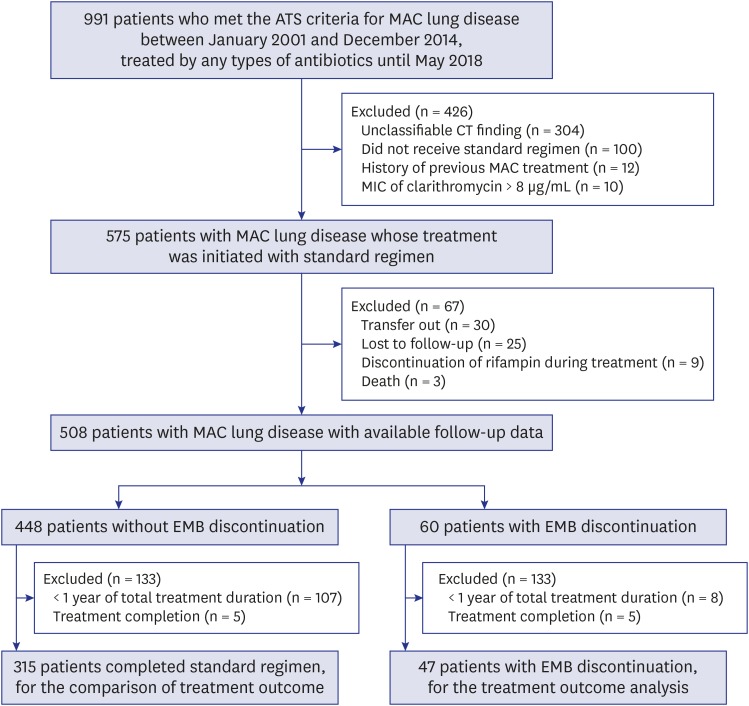
A retrospective review of medical records between January 2001 and December 2014 at the Asan Medical Center, a 2,700-bed referral hospital in Seoul, Korea, revealed 991 patients who fulfilled the American Thoracic Society diagnostic criteria for MAC-LD. Of these, we identified 575 patients with MAC-LD who received at least one dose of the standard treatment. Of these 575 patients, we excluded those who were transferred to another hospital, were lost to follow-up, or died due to any reasons, as well as the patients who discontinued rifamycin due to various adverse effects. After these exclusions, 508 patients with MAC-LD whose follow-up data were available remained for analysis (Fig. 1).
Fig. 1. Study flow chart.
ATS = American Thoracic Society, MAC = Mycobacterium avium complex, CT = computed tomography, MIC = minimum inhibitory concentration, EMB = ethambutol.
Treatment outcome analysis
Sputum culture conversion was defined as the achievement of three consecutive negative sputum cultures, and the date of the first negative culture was defined as the date of culture conversion.7 The treatment outcome analysis was performed for patients who received the treatment for at least ≥ 12 months, after excluding whose total treatment duration was < 12 months. This is because recent studies concerning treatment outcome analysis of MAC-LD have only included patients who received the therapy for ≥ 12 months,8,9,10 although debate exists regarding the exclusion of patients who received the treatment for < 12 months from the analysis.11 The treatment outcomes were categorized into success, failure, and completion.9,10,12 Treatment success was defined as achievement of culture conversion with treatment duration lasting at least 12 months after the date of the first culture conversion. Treatment failure was defined as no conversion to negative sputum culture even after ≥ 12 months of treatment. Treatment completion was defined as achievement of culture conversion but with treatment duration of < 12 months after the date of the first culture conversion. For the treatment outcome analysis, we selected only patients whose outcome was treatment success or treatment failure and excluded patients with treatment completion, according to treatment outcome analysis in our previous studies.9,10,12 In the remaining patients, we analyzed the treatment outcome of the patients with EMB discontinuation by comparing their outcomes with those of matched subjects who received standard treatment regimen without EMB discontinuation during the same period in our center (Fig. 1).
Microbiological examination and radiologic evaluation
Acid-fast bacillus (AFBs) smears were identified by Ziehl–Neelsen staining. Solid (Ogawa medium; Korean Institute of Tuberculosis, Seoul, Korea) and liquid (BACTEC 960 Mycobacterial Growth Indicator Tube; Becton Dickinson, Sparks, MD, USA) media were used to culture AFB. Polymerase chain reaction (PCR) assay using Seeplex TB detection (Seegen, Seoul, Korea) was used to differentiate between Mycobacterium tuberculosis complex and NTM and then, the NTM species were identified by PCR and restriction fragment length polymorphism methods, using the rpoB gene.13
Radiological findings from computed tomography of the chest were used to categorize disease type into fibrocavitary, cavitary nodular bronchiectatic (NB) and noncavitary NB forms.8,14
Statistical analysis
The data were compared using a Student's t-test for the continuous variables and a χ2 or Fisher's exact test for the categorical variables. For comparison of the treatment outcomes between the patients with EMB discontinuation and those who completed standard treatment, we used propensity scores calculated by logistic regression to adjust for between-group differences. Positive AFB smears at treatment initiation and radiologic types were included in the propensity model, because these two variables are most relevant to the treatment outcome of MAC-LD. Using propensity score matching, the two groups were matched in a 1:4 ratio. We used standardized mean differences to compare the two groups after the propensity score matching. All tests for statistical significance were two-sided, and P values of < 0.05 were considered to indicate statistical significance. All analyses were performed using R (3.5.1 version; R Foundation, Vienna, Austria) and SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB No. 2019-0748). The requirement for informed consent was waived because of the retrospective nature of the analysis.
RESULTS
Study subjects
Among the 508 patients who initiated standard treatment regimen, 60 (11.8%) patients discontinued EMB due to adverse events. Table 1 presents the baseline characteristics of these 60 patients. The mean age was 64.4 ± 11.0 years with a preponderance of women patients (60.0%). The most common radiographic finding was noncavitary NB type (45.0%, 27/60), followed by cavitary NB (33.3%, 20/60) and fibrocavitary type (21.7%, 13/60). The majority of patients (96.7%, 58/60) received daily therapy. The mean duration of EMB administration before discontinuation was 7.0 ± 4.6 months, which accounted for 48.1% of the total treatment duration. EMB-related optic neuropathy was the most common cause of discontinuation of EMB, being identified in 45 of the 60 patients (75.0%). In the remaining 15 patients, the causes of EMB discontinuation were skin rash (8.4%, 5/60), gastrointestinal disturbance (6.7%, 4/60), hepatotoxicity (3.3%, 2/60), or dizziness and headache (3.3%, 2/60), and causes were unrecorded in the remaining patients (3.3%, 2/60).
Table 1. Baseline characteristics of 60 patients with EMB discontinuation.
| Characteristics | Patients with EMB discontinuation (n = 60) | |
|---|---|---|
| Age, yr | 64.4 ± 11.0 | |
| Age ≥ 60 yr | 41 (68.3) | |
| Gender, women | 36 (60.0) | |
| Body mass index, kg/m2 | 19.5 ± 3.0 | |
| Current or past smoker | 19 (31.7) | |
| Previous history of TB treatment | 27 (45.0) | |
| Comorbidities | ||
| Malignancy | 18 (30.0) | |
| Diabetes mellitus | 6 (10.0) | |
| COPD | 5 (8.3) | |
| Etiology | ||
| Mycobacterium avium | 29 (48.3) | |
| Mycobacterium intracellulare | 31 (51.7) | |
| Type of disease | ||
| Noncavitary NB | 27 (45.0) | |
| Cavitary NB | 20 (33.3) | |
| Fibrocavitary | 13 (21.7) | |
| Positive AFB smear | 34 (56.7) | |
| Use of injectable aminoglycoside | 38 (63.3) | |
| Duration of EMB administration, mon | 7.0 ± 4.6 | |
Data are presented as mean ± standard deviation or number (%).
EMB = ethambutol, TB = tuberculosis, COPD = chronic obstructive pulmonary disease, NB = nodular bronchiectatic, AFB = acid-fast bacilli.
Within the group of 60 patients with EMB discontinuation, treatment outcome analysis was performed in 47 patients (Fig. 1). These patients were matched by propensity score with patients who completed the standard regimen.
Treatment regimens after EMB discontinuation
Table 2 shows the detailed treatment regimens and their duration in the 47 patients with EMB discontinuation for whom treatment outcome analysis was performed. After discontinuation of EMB, patients received one of three treatment regimens. First, 48.9% (23/47) of patients were treated with macrolide, rifampin and later-generation fluoroquinolone (mostly moxifloxacin) instead of EMB for the remaining treatment period. Their mean total treatment duration was 16.1 ± 3.3 months. These patients received an EMB-containing regimen for 6.0 ± 4.5 months (which accounted for 42.1% of the total treatment duration), and were then treated for the remaining 10.1 ± 4.5 months (57.9% of the total duration) with the fluoroquinolone-containing regimen. Second, 38.3% (18/47) of patients were treated with macrolide and rifampin without any additional drugs. In these 18 patients, EMB was administered for 9.6 ± 5.2 months (52.4% of the total treatment duration) before discontinuation. Third, the remaining 6 (12.8%) patients received various antibiotics instead of EMB, including ciprofloxacin (n = 3), clofazimine (n = 2), and isoniazid (n = 1).
Table 2. Detailed regimens and treatment duration in 47 patients with EMB discontinuation.
| Regimen after EMB discontinuation | Total treatment duration, mon | Duration of EMB administration, mon | EMB administration for total treatment period, % |
|---|---|---|---|
| Total patients (n = 47) | 17.2 ± 3.4 | 7.3 ± 4.9 | 42.1 ± 25.8 |
| Macrolide, rifampin, later-generation FQ (n = 23) | 16.1 ± 3.3 | 6.0 ± 4.5 | 36.5 ± 26.3 |
| Macrolide, rifampin (n = 18) | 18.3 ± 3.7 | 9.6 ± 5.2 | 52.4 ± 24.4 |
| Macrolide, rifampin, other antibioticsa (n = 6) | 18.0 ± 2.0 | 5.7 ± 3.3 | 32.8 ± 20.4 |
Data are presented as mean ± standard deviation.
EMB = ethambutol, FQ = fluoroquinolone.
aAmong six patients who received other antibiotics, thee patients were treated with ciprofloxacin, two patients were treated with clofazimine, and one patient was treated with isoniazid.
Treatment outcome
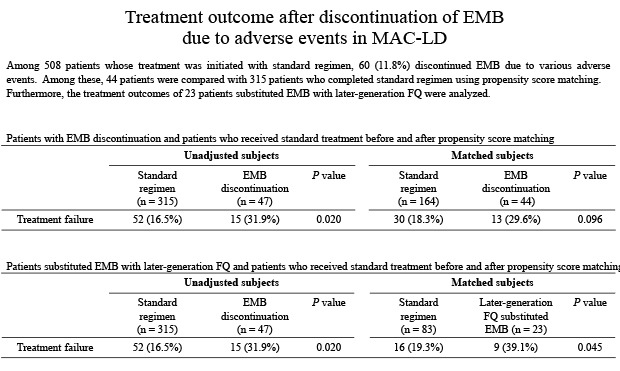
After the propensity score adjusted matching, 44 (93.6%) of 47 patients with EMB discontinuation were compared with 164 (52.1%) out of 315 patients who completed standard treatment. The reason for the exclusion of three patients with EMB discontinuation from propensity score-adjusted matching was that these patients' baseline variables could not be matched to those of the patients who received standard treatment. Table 3 shows the baseline characteristics and treatment outcome of the patients before and after propensity matching. After propensity matching, the treatment failure rate was 18.3% (30/164) in patients who completed the standard regimen and 29.6% (13/44) in those with EMB discontinuation. Although the treatment outcome tended to be poorer in patients with EMB discontinuation, there was no statistically significant difference between the two groups (P = 0.095).
Table 3. Comparison of baseline characteristics and treatment outcome between patients who received standard treatment and patients with EMB discontinuation according to detailed regimens before and after propensity score matching.
| Characteristics | Unadjusted subjects | Matched subjects | Matched subjects | Matched subjects | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard regimen (n = 315) | EMB discontinuation (n = 47) | P value | Standard regimen (n = 164) | EMB discontinuationa (n = 44) | SMD | Standard regimen (n = 83) | Later-generation FQsb (n = 23) | SMD | Standard regimen (n = 68) | Macrolide, rifampinc (n = 17) | SMD | ||
| Age, yr | 59.9 ± 10.5 | 64.2 ± 11.6 | 0.010 | 61.0 ± 10.1 | 63.8 ± 10.9 | 0.266 | 60.8 ± 10.5 | 65.3 ± 10.8 | 0.420 | 60.5 ± 11.6 | 63.5 ± 10.9 | 0.268 | |
| Age ≥ 60 yr | 164 (52.1) | 33 (70.2) | 0.030 | 92 (56.1) | 30 (68.2) | 0.251 | 45 (54.2) | 17 (73.9) | - | 36 (52.9) | 11 (64.7) | 0.241 | |
| Gender, women | 201 (63.8) | 29 (61.7) | 0.906 | 96 (58.5) | 26 (59.1) | 0.011 | 49 (59.0) | 11 (47.8) | 0.226 | 38 (55.9) | 13 (76.5) | 0.446 | |
| Body mass index, kg/m2 | 20.7 ± 2.5 | 19.2 ± 3.0 | < 0.001 | 20.5 ± 2.4 | 19.2 ± 3.1 | 0.469 | 20.6 ± 2.3 | 19.7 ± 3.4 | 0.296 | 20.6 ± 2.7 | 18.8 ± 2.7 | 0.646 | |
| Current or past smoker | 84 (26.7) | 12 (25.5) | > 0.999 | 53 (32.3) | 12 (27.3) | 0.110 | 25 (30.1) | 8 (34.8) | 0.100 | 22 (32.4) | 3 (17.7) | 0.345 | |
| Previous history of TB treatment | 131 (41.6) | 23 (48.9) | 0.428 | 80 (48.8) | 23 (52.3) | 0.070 | 37 (44.6) | 12 (52.2) | 0.152 | 32 (47.1) | 8 (47.1) | 0.000 | |
| Comorbidities | |||||||||||||
| Malignancy | 58 (18.4) | 15 (31.9) | 0.050 | 33 (20.1) | 15 (34.1) | 0.318 | 18 (21.7) | 11 (47.8) | 0.517 | 16 (23.5) | 4 (23.5) | 0.000 | |
| COPD | 43 (13.6) | 4 (8.5) | 0.456 | 22 (13.4) | 4 (9.1) | 0.137 | 13 (15.7) | 3 (13.0) | 0.075 | 11 (16.2) | 1 (5.9) | 0.333 | |
| Diabetes mellitus | 28 (8.9) | 6 (12.8) | 0.561 | 13 (7.9) | 5 (11.4) | 0.117 | 9 (10.8) | 3 (13.0) | 0.068 | 5 (7.4) | 1 (5.9) | 0.059 | |
| Etiology | 0.369 | 0.113 | 0.104 | 0.029 | |||||||||
| Mycobacterium avium | 160 (50.8) | 20 (42.6) | 80 (48.8) | 19 (43.2) | 44 (53.0) | 11 (47.8) | 33 (48.5) | 8 (47.1) | |||||
| Mycobacterium intracellulare | 155 (49.2) | 27 (57.4) | 84 (52.2) | 25 (56.8) | 39 (47.0) | 12 (52.2) | 35 (51.5) | 9 (52.9) | |||||
| Type of disease | 0.002 | 0.080 | 0.114 | 0.000 | |||||||||
| Noncavitary NB | 223 (70.8) | 21 (44.7) | 84 (51.2) | 21 (47.7) | 36 (43.4) | 9 (39.1) | 20 (29.4) | 5 (29.4) | |||||
| Cavitary NB | 54 (17.1) | 16 (34.0) | 43 (26.2) | 13 (29.6) | 35 (42.2) | 11 (47.8) | 36 (52.9) | 9 (52.9) | |||||
| Fibrocavitary | 38 (12.1) | 10 (21.3) | 37 (22.6) | 10 (22.7) | 12 (14.4) | 3 (13.1) | 12 (17.7) | 3 (17.7) | |||||
| Positive AFB smear | 125 (39.7) | 27 (57.5) | 0.032 | 87 (53.1) | 24 (54.6) | 0.030 | 47 (56.6) | 14 (60.9) | 0.086 | 28 (41.2) | 7 (41.2) | 0.000 | |
| Use of injectable aminoglycoside | 150 (47.6) | 30 (63.8) | 0.055 | 85 (51.8) | 28 (63.6) | 0.241 | 45 (54.22) | 11 (47.8) | 0.128 | 40 (58.8) | 12 (70.1) | 0.248 | |
| Treatment failured | 52 (16.5) | 15 (31.9) | 0.020 | 30 (18.3) | 13 (29.6) | - | 16 (19.3) | 9 (39.1) | - | 9 (13.2) | 3 (17.7) | - | |
Data are presented as mean ± standard deviation or number (%).
EMB = ethambutol, SMD = standardized mean differences, FQ = fluoroquinolone, TB = tuberculosis, COPD = chronic obstructive pulmonary disease, NB = nodular bronchiectatic, AFB = acid-fast bacilli.
aAmong 47 patients with EMB discontinuation, 44 patients were matched with 164 out of 315 patients who received the standard regimen; bAll 23 patients treated with later-generation FQs were compared with 83 of 315 patients who received the standard regimen; cAmong 18 patients treated with macrolide and rifampin without any additional drugs, 17 patients were matched with 68 of 315 patients who received the standard regimen; dSMD value represented the difference in baseline variables between two groups after propensity score-adjusted matching. Because the treatment failure is the outcome value, the treatment failure did not have the SMD value.
We further analyzed the treatment outcomes of 23 patients treated with later-generation fluoroquinolone instead of EMB. The treatment failure rate of these 23 patients was significantly higher than that of 83 matched patients who completed the standard regimen (39.1% vs. 19.3%, P = 0.045).
Of 18 patients treated with macrolide and rifampin without any additional drugs, 17 patients were matched with 68 patients who completed the standard regimen (Table 3). Remaining one patient was excluded from propensity score-adjusted matching because this patient's baseline variables could not be matched to those of the patients who received standard treatment. The treatment failure rate of these 17 patients was 17.7%, which was similar to those who completed the standard regimen (13.2% vs. 17.7%, P = 0.645).
DISCUSSION
Although EMB is the second most important drug in the treatment of MAC-LD, it is not rare for this drug to be unexpectedly discontinued in clinical practice because of its adverse events. To date, there has been no study concerning treatment outcomes in this clinical situation. This is the first study to investigate treatment outcomes of the various regimens following discontinuation of EMB due to adverse events. Our findings reveal several clinical implications. First, it was not uncommon for patients to require discontinuation of EMB because of various adverse events. Second, the overall treatment failure rate of patients with EMB discontinuation was relatively high compared with that of those who completed the standard regimen without EMB discontinuation, although statistical significance was not found. Third, there was a statistically significant high failure rate in patients who were prescribed later-generation fluoroquinolones to replace EMB.
The most common reason for EMB discontinuation in our study subjects was optic neuropathy. Optic neuropathy is a dose-dependent adverse effect of EMB administration, occurring in approximately 5%–6% of patients receiving a dosage of 25 mg/kg/day, and in 1% of patients receiving a dosage of 15 mg/kg/day or less.15 One report showed that 5.8% (8/139) of patients on daily therapy were diagnosed with EMB-related ocular toxicity after a mean duration of 16.1 ± 10.8 months of multidrug therapy, which included EMB,5 which was similar to the rate observed in the present study (8.9%, 45/508). In another study, EMB was discontinued in 24% (24/99) of patients in a daily therapy group, but the exact incidence of EMB-related ocular toxicity was not reported.16 EMB-related ocular toxicity appears to be higher in patients with MAC-LD than in those with tuberculosis, based on the findings of one study in which an EMB-related visual disturbance occurred in 1.8% (9/492) of patients with tuberculosis.17 This might be due to the general older age and longer treatment duration in patients with MAC-LD. After optic neuropathy, skin rash and gastrointestinal disturbance were the most common reasons for EMB discontinuation in the subjects of the present study.
It is well known that treatment outcomes of MAC-LD patients are relatively poor, with a treatment success rate of only 60%.18 In contrast, the reported outcome of patents who receive ≥ 12 months of treatment is more favorable. For instance, one study reported an unfavorable outcome of 12% in noncavitary NB group, and 22%–24% in the cavitary group.8 Moreover, we previously showed that the overall treatment failure rate was 20.4% (56/274) in patients with MAC-LD including the cavitary and noncavitary type.9 Compared with the treatment failure rate of these previous studies, the overall treatment failure rate in the present study appeared to be higher. The 44 patients with EMB discontinuation had a tendency for higher treatment failure rate than 164 matched control patients, although this difference was not statistically significant. As the number of patients with EMB discontinuation was relatively small, we thought that an analysis of higher numbers of patients with EMB discontinuation may reveal statistical significance. These findings suggest discontinuation of EMB without appropriate alternatives may lead to a poor outcome and support the importance of EMB (second only to macrolides) in the present drug regimens for treating MAC-LD.
Another important implication of the present study is that the treatment failure rate even in patients whose EMB treatment was replaced with later-generation fluoroquinolone was unacceptable. In drug-susceptible pulmonary tuberculosis, moxifloxacin shows stronger anti-tuberculosis activity than EMB,19 and in addition, later-generation fluoroquinolone is one of the three core drugs for treating multidrug resistant tuberculosis.20 In the context of tuberculosis, later-generation fluoroquinolone can be prescribed as an alternative to EMB if necessary due to adverse events.21 In contrast, the treatment effect of fluoroquinolone is reported to be low in MAC-LD. Indeed, there is no convincing evidence that fluoroquinolones are effective for treating MAC, despite being used for its treatment for more than 2 decades.22 In the present study, we showed that the treatment failure rate in 23 patients treated with later-generation fluoroquinolone after EMB discontinuation was significantly higher than the treatment failure rate of the matched patients. If fluoroquinolones indeed had a significant therapeutic effect against MAC-LD, we would expect to find that treatment failure rates in fluoroquinolone-treated patients were similar to controls. Therefore, when EMB discontinuation is needed because of adverse events during MAC-LD treatment, we cautiously suggest that it would be better for the attending physician to 1) prescribe one or more other potential drugs instead of later-generation fluoroquinolones, or 2) consider prescribing one or more other drugs in addition to later-generation fluoroquinolones to improve treatment outcomes. Further research is needed to elucidate the optimal regimen after EMB discontinuation.
In the present study, three patients receiving ciprofloxacin were not integrated into the “fluoroquinolone” group, along with those receiving later-generation fluoroquinolones, but they were classified into the “other antibiotics” group (Table 2). This is because previous studies have shown that the efficacy of ciprofloxacin for MAC-LD treatment is not prominent.23,24 Meanwhile, moxifloxacin has been reported to provide more favorable outcomes in various in vitro and in vivo models.25,26,27 However, notably, there has been no study to directly compare the efficacy of ciprofloxacin and later-generation fluoroquinolones, such as moxifloxacin, against MAC-LD.
A total of 38.3% (18/47) patients were treated with only macrolide and rifampin after discontinuation of EMB, without the addition of other drugs. The treatment outcome of these patients was comparable to that of those who completed the standard regimen, despite the discontinuation of the second most important drug, EMB. The reason for this unexpectedly similar outcome is unclear, but may be due to a relatively longer treatment duration of EMB before discontinuation in the patients treated only with macrolide and rifampin (9.6 ± 5.2 months, 52.4% of the total treatment duration) compared with the other patients who discontinued EMB (5.9 ± 4.3 months, 35.7% of the total treatment duration, P = 0.029 compared with patients treated only with macrolide and rifampin). It is important to note that we do not believe our findings mean that this treatment option should be chosen by an attending physician in clinical practice, particularly when EMB discontinuation is necessary earlier in MAC-LD treatment, as previous reports have consistently shown that a combination therapy of only macrolide and rifampin without EMB can induce macrolide resistance in patients with MAC-LD.28,29 That is, if a patient is treated with macrolide and rifampin without additional drug after EMB discontinuation, it is possible that the patient could develop a macrolide-resistant strain.
Our study has several limitations. First, this was a single center retrospective study performed with a relatively small sample size. Second, we could not adjust for the possible effects of the duration of EMB use before discontinuation on the subsequent treatment outcome. Lastly, a small number of patients with EMB discontinuation were not selected for the propensity score matching.
In conclusion, we showed that it was not uncommon for patients being treated for MAC-LD to discontinue EMB due to adverse events. The treatment outcome of these patients tended to be generally unsatisfactory, although there was not a statistical difference compared with matched control patients who completed the standard regimen. In addition, it is notable that the treatment failure rate was significantly higher in the patients who received later-generation fluoroquinolones as a substitute for EMB. These findings suggest that the attending physician could consider prescribing one or more drugs in addition to or instead of fluoroquinolone when EMB discontinuation is needed during the treatment of MAC-LD.
ACKNOWLEDGMENTS
The authors would like to thank Minkyu Han, Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, for statistical analysis and his contribution to the manuscript.
Footnotes
Funding: This work was supported by the National Research Foundation of Korea grant funded by the Korea government (Ministry of Science and ICT) (No. 2019R1F1A1059190).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Shim TS, Jo KW, Chong YP.
- Data curation: Kwon YS, Kwon BS, Kim OH, Park YE.
- Formal analysis: Kwon YS.
- Investigation: Jo KW, Kwon YS.
- Methodology: Jo KW, Shim TS.
- Writing - original draft: Jo KW, Kwon YS.
- Writing - review & editing: Shim TS, Jo KW, Chong YP.
References
- 1.Kwon YS, Koh WJ. Diagnosis and treatment of nontuberculous mycobacterial lung disease. J Korean Med Sci. 2016;31(5):649–659. doi: 10.3346/jkms.2016.31.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko RE, Moon SM, Ahn S, Jhun BW, Jeon K, Kwon OJ, et al. Changing epidemiology of nontuberculous mycobacterial lung diseases in a tertiary referral hospital in Korea between 2001 and 2015. J Korean Med Sci. 2018;33(8):e65. doi: 10.3346/jkms.2018.33.e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon YS, Koh WJ, Daley CL. Treatment of Mycobacterium avium complex pulmonary disease. Tuberc Respir Dis. 2019;82(1):15–26. doi: 10.4046/trd.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 5.Griffith DE, Brown-Elliott BA, Shepherd S, McLarty J, Griffith L, Wallace RJ., Jr Ethambutol ocular toxicity in treatment regimens for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2005;172(2):250–253. doi: 10.1164/rccm.200407-863OC. [DOI] [PubMed] [Google Scholar]
- 6.Kamii Y, Nagai H, Kawashima M, Matsuki M, Nagoshi S, Sato A, et al. Adverse reactions associated with long-term drug administration in Mycobacterium avium complex lung disease. Int J Tuberc Lung Dis. 2018;22(12):1505–1510. doi: 10.5588/ijtld.18.0171. [DOI] [PubMed] [Google Scholar]
- 7.van Ingen J, Aksamit T, Andrejak C, Böttger EC, Cambau E, Daley CL, et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur Respir J. 2018;51(3):1800170. doi: 10.1183/13993003.00170-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh WJ, Moon SM, Kim SY, Woo MA, Kim S, Jhun BW, et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur Respir J. 2017;50(3):1602503. doi: 10.1183/13993003.02503-2016. [DOI] [PubMed] [Google Scholar]
- 9.Kwon BS, Kim MN, Sung H, Koh Y, Kim WS, Song JW, et al. In vitro MIC values of rifampin and ethambutol and treatment outcome in Mycobacterium avium complex lung disease. Antimicrob Agents Chemother. 2018;62(10):e00491-18. doi: 10.1128/AAC.00491-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim OH, Kwon BS, Han M, Koh Y, Kim WS, Song JW, et al. Association between duration of aminoglycoside treatment and outcome of cavitary Mycobacterium avium complex lung disease. Clin Infect Dis. 2019;68(11):1870–1876. doi: 10.1093/cid/ciy804. [DOI] [PubMed] [Google Scholar]
- 11.Loebinger MR. Mycobacterium avium complex infection: phenotypes and outcomes. Eur Respir J. 2017;50(3):1701380. doi: 10.1183/13993003.01380-2017. [DOI] [PubMed] [Google Scholar]
- 12.Kwon BS, Shim TS, Jo KW. The second recurrence of MAC lung disease after successful treatment for first recurrence. Eur Respir J. 2019;53(1):1801038. doi: 10.1183/13993003.01038-2018. [DOI] [PubMed] [Google Scholar]
- 13.Adékambi T, Drancourt M, Raoult D. The rpoB gene as a tool for clinical microbiologists. Trends Microbiol. 2009;17(1):37–45. doi: 10.1016/j.tim.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J. 2017;49(3):1600537. doi: 10.1183/13993003.00537-2016. [DOI] [PubMed] [Google Scholar]
- 15.Wang MY, Sadun AA. Drug-related mitochondrial optic neuropathies. J Neuroophthalmol. 2013;33(2):172–178. doi: 10.1097/WNO.0b013e3182901969. [DOI] [PubMed] [Google Scholar]
- 16.Jeong BH, Jeon K, Park HY, Kim SY, Lee KS, Huh HJ, et al. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015;191(1):96–103. doi: 10.1164/rccm.201408-1545OC. [DOI] [PubMed] [Google Scholar]
- 17.Carroll MW, Lee M, Cai Y, Hallahan CW, Shaw PA, Min JH, et al. Frequency of adverse reactions to first- and second-line anti-tuberculosis chemotherapy in a Korean cohort. Int J Tuberc Lung Dis. 2012;16(7):961–966. doi: 10.5588/ijtld.11.0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwak N, Park J, Kim E, Lee CH, Han SK, Yim JJ. Treatment outcomes of Mycobacterium avium complex lung disease: a systematic review and meta-analysis. Clin Infect Dis. 2017;65(7):1077–1084. doi: 10.1093/cid/cix517. [DOI] [PubMed] [Google Scholar]
- 19.Conde MB, Efron A, Loredo C, De Souza GR, Graça NP, Cezar MC, et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet. 2009;373(9670):1183–1189. doi: 10.1016/S0140-6736(09)60333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO consolidated guidelines on drug-resistant tuberculosis treatment. [Update 2019]. [Accessed June 1, 2019]. https://apps.who.int/iris/bitstream/handle/10665/311389/9789241550529-eng.pdf.
- 21.Fouad M, Gallagher JC. Moxifloxacin as an alternative or additive therapy for treatment of pulmonary tuberculosis. Ann Pharmacother. 2011;45(11):1439–1444. doi: 10.1345/aph.1Q299. [DOI] [PubMed] [Google Scholar]
- 22.Griffith DE. Macrolide-resistant Mycobacterium avium complex: “I feel like I'e been here before”. Ann Am Thorac Soc. 2016;13(11):1881–1882. doi: 10.1513/AnnalsATS.201609-666ED. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins PA, Campbell IA, Banks J, Gelder CM, Prescott RJ, Smith AP. Clarithromycin vs ciprofloxacin as adjuncts to rifampicin and ethambutol in treating opportunist mycobacterial lung diseases and an assessment of Mycobacterium vaccae immunotherapy. Thorax. 2008;63(7):627–634. doi: 10.1136/thx.2007.087999. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson MA, Yajko D, Northfelt D, Charlebois E, Gary D, Brosgart C, et al. Randomized, placebo-controlled trial of rifampin, ethambutol, and ciprofloxacin for AIDS patients with disseminated Mycobacterium avium complex infection. J Infect Dis. 1993;168(1):112–119. doi: 10.1093/infdis/168.1.112. [DOI] [PubMed] [Google Scholar]
- 25.Bermudez LE, Inderlied CB, Kolonoski P, Petrofsky M, Aralar P, Wu M, et al. Activity of moxifloxacin by itself and in combination with ethambutol, rifabutin, and azithromycin in vitro and in vivo against Mycobacterium avium . Antimicrob Agents Chemother. 2001;45(1):217–222. doi: 10.1128/AAC.45.1.217-222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bermudez LE, Kolonoski P, Petrofsky M, Wu M, Inderlied CB, Young LS. Mefloquine, moxifloxacin, and ethambutol are a triple-drug alternative to macrolide-containing regimens for treatment of Mycobacterium avium disease. J Infect Dis. 2003;187(12):1977–1980. doi: 10.1086/375352. [DOI] [PubMed] [Google Scholar]
- 27.Sano C, Tatano Y, Shimizu T, Yamabe S, Sato K, Tomioka H. Comparative in vitro and in vivo antimicrobial activities of sitafloxacin, gatifloxacin and moxifloxacin against Mycobacterium avium. Int J Antimicrob Agents. 2011;37(4):296–301. doi: 10.1016/j.ijantimicag.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Morimoto K, Namkoong H, Hasegawa N, Nakagawa T, Morino E, Shiraishi Y, et al. Macrolide-resistant Mycobacterium avium complex lung disease: analysis of 102 consecutive cases. Ann Am Thorac Soc. 2016;13(11):1904–1911. doi: 10.1513/AnnalsATS.201604-246OC. [DOI] [PubMed] [Google Scholar]
- 29.Moon SM, Park HY, Kim SY, Jhun BW, Lee H, Jeon K, et al. Clinical characteristics, treatment outcomes, and resistance mutations associated with macrolide-resistant Mycobacterium avium complex lung disease. Antimicrob Agents Chemother. 2016;60(11):6758–6765. doi: 10.1128/AAC.01240-16. [DOI] [PMC free article] [PubMed] [Google Scholar]