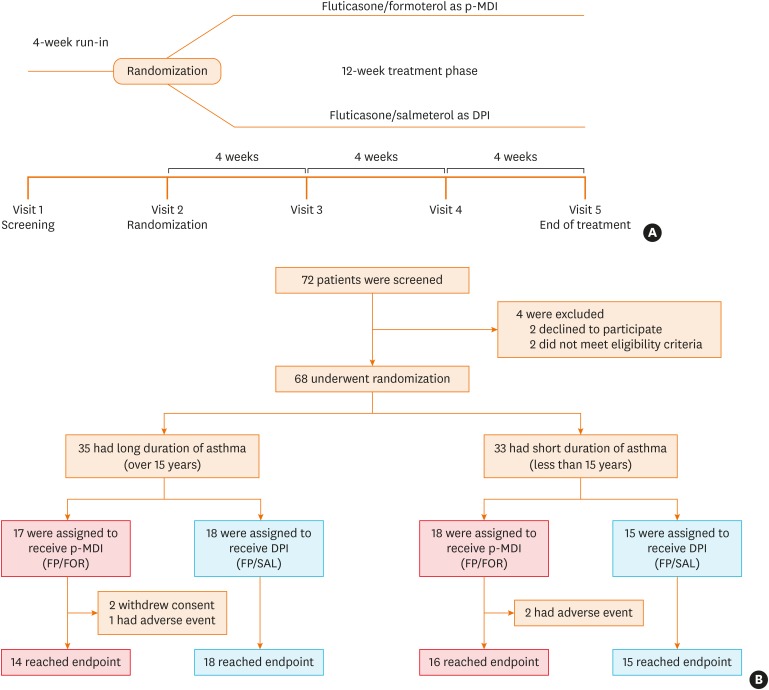
Fig. 1. Trial design and CONSORT flow.
(A) shows the design of the trial and (B) shows the screening, randomization, and treatment for patients stratified according to asthma duration (≥ 15 years or < 15 years). All patients who underwent randomization into the treatment method were included in the full analysis set population.
CONSORT, Consolidated Standards Of Reporting Trials; p-MDI, pressurized metered-dose inhalers; FP/FOR, fluticasone propionate/formoterol fumarate; DPI, dry powder inhalers; FP/SAL, fluticasone propionate/salmeterol xinafoate.