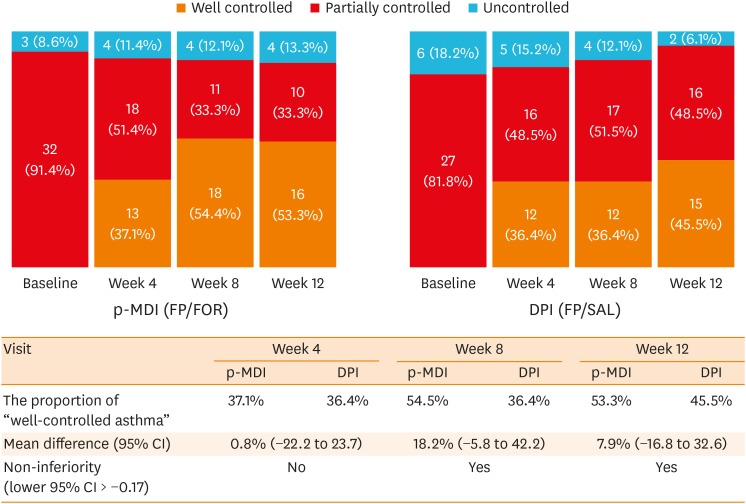
Fig. 2. The proportion of asthma control status in the p-MDI and DPI groups during the study period.
Asthma control status was assessed according to the GINA guidelines. The proportion test (one-sided) for proving non-inferiority of the FP/FOR group and the FP/SAL group in the PP population, with a predetermined non-inferiority margin of −17.0% for the difference in the rates of well-controlled asthma between the 2 groups. PP principle was applied to reduce possible statistical bias caused by missing data imputation.
p-MDI, pressurized metered-dose inhalers; FP/FOR, fluticasone propionate/formoterol fumarate; DPI, dry powder inhalers; FP/SAL, fluticasone propionate/salmeterol xinafoate; GINA, Global Initiative for Asthma; PP, per-protocol; CI, confidence interval.